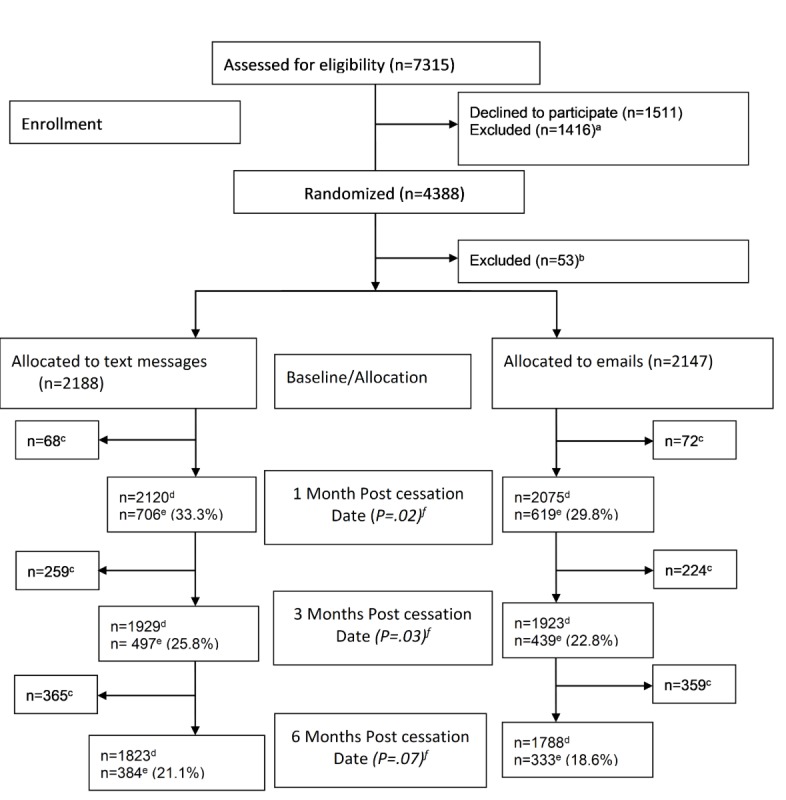
Consolidated Standards of Reporting Trials’ diagram. Randomized controlled trial, Norway 2010-2012 (N= 4335). a – already stopped smoking (n=631); did not complete baseline registration (n=517); not smoking cigarettes (n=20); referred to substudy (n=248). b – text message/email arm; consent withdrawn n=29 (17/12); missing/double allocation n=24 (12/12). c – participants that had not completed the next follow-up time point. d – Participants included in the analysis. e – responders to follow-up email questionnaire. f – Chi-square statistics; P value for difference between the 2 arms.
