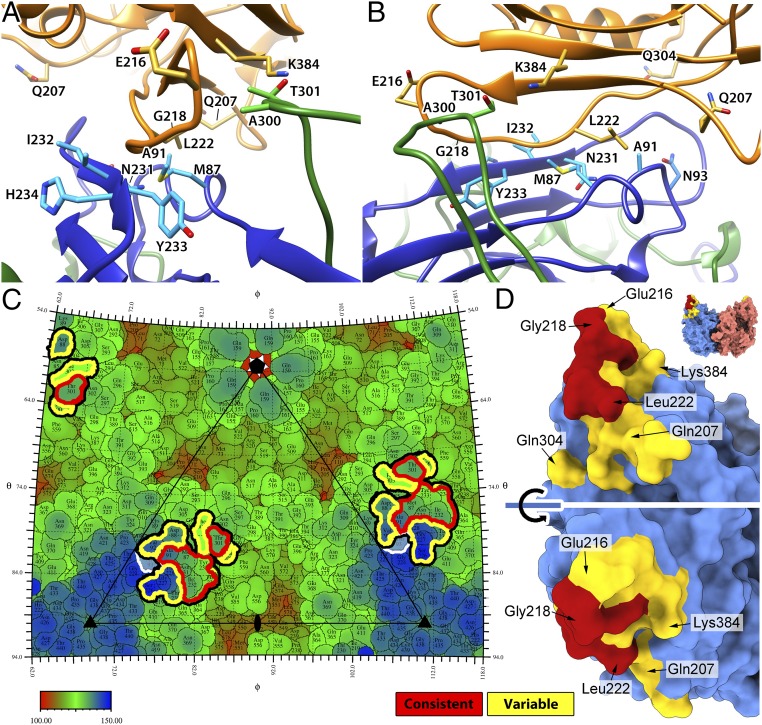
Fig. 7.
The fitting of the TfR structure identifies the dynamic interactions between CPV and TfR. (A and B) The refined bbj-TfR structure was fitted into the 5 cryo-EM maps. Fitted structures for class 3 are depicted. The 2 CPV VP2 chains and TfR are shown as ribbon diagrams and colored in blue, green, and orange, respectively. Side chains of the consistent contacts as well as Asn-93 and Ala-300 of CPV residues and TfR residues 216, 218, 207, 222, 304, and 384 are shown as stick models and labeled. (C) The roadmap of the CPV capsid surface with TfR footprint indicated. All of the receptor-contacting residues are highlighted by thick black lines. The consistent and variable contacts are further highlighted in red and yellow lines, respectively. CPV residue 93 is marked by white lines. The capsid was radially colored according to the color map, and 1 asymmetric unit is indicated by a triangle with icosahedral symmetry axis marks. (D) The capsid-contacting residues on the model of the bbj-TfR molecule. The TfR was surface rendered, and each monomer was colored differently (top). The residues that are seen to make consistent and variable contacts with the capsid were colored red and yellow, respectively. The large red surface area includes residues from the βII-1/βII-2 loop in addition to Val-213 and Leu-222.