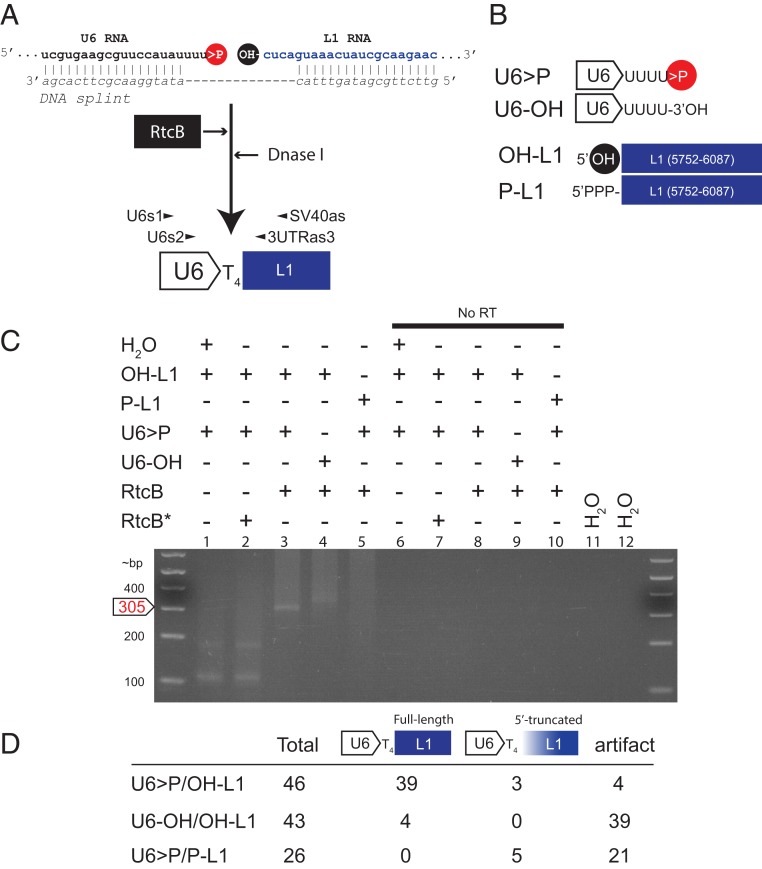
Fig. 2.
Purified recombinant RtcB ligates U6 RNA to L1 RNA in vitro. (A) The rationale of the U6/L1 in vitro ligation experiment. A synthetic human U6 RNA containing a 2′,3′-cyclic phosphate (>P, red circle) and a synthetic L1 RNA (blue font) containing a 5′-OH (black circle) were generated using a ribozyme-based in vitro transcription reaction. U6 and L1 RNAs were splinted with a cDNA oligonucleotide (DNA splint, italics), and then the resultant RNA/DNA hybrid was incubated with purified RtcB (black rectangle, white font) for 1 h. The reaction was then treated with DNase I, the RNA was purified, and cDNAs were synthesized using the SV40as oligonucleotide primer. RT-PCR reactions using nested primers (U6s1 and SV40as, then U6s2 and 3UTRas3) were used to detect U6/L1 chimeric cDNAs. (B) Schematic representations of the synthetic RNAs used in in vitro experiments. The in vitro transcribed U6 RNA ends in 4 uridine ribonucleotides and contains a 2′,3′-cyclic phosphate (U6 > P) or a 3′-OH (U6-OH). The in vitro transcribed L1 RNA consists of pJM101/L1.3Δneo sequence (nt positions 5752 to 6087) and contains a 5′-OH (OH-L1) or a 5′-triphosphate (P-L1). (C) Results from the in vitro U6/L1 ligation reactions. The constituents of U6/L1 ligation reactions are indicated above each gel lane (+) of the agarose gel image. An asterisk (*) indicates that RtcB was heat treated at 95 °C for 10 min prior to adding it to the reaction. No RT, no RT control; H2O, water PCR controls. DNA size markers (in bp) are shown to the left of the gel image. The predicted position of the 305-bp U6/L1 RT-PCR product is noted on the left side of the gel image (white arrow, red font). (D) Summary of results from product characterization experiments. Column 1, synthetic RNAs used in the reaction; column 2, number of RT-PCR products characterized for each reaction condition; column 3, number of RT-PCR products that correspond to the full-length ligation product; column 4, number of RT-PCR products that contain a variably 5′-truncated L1 sequence; column 5, number of putative RT-PCR artifact products. Each in vitro experiment was repeated 3 independent times and yielded similar results.