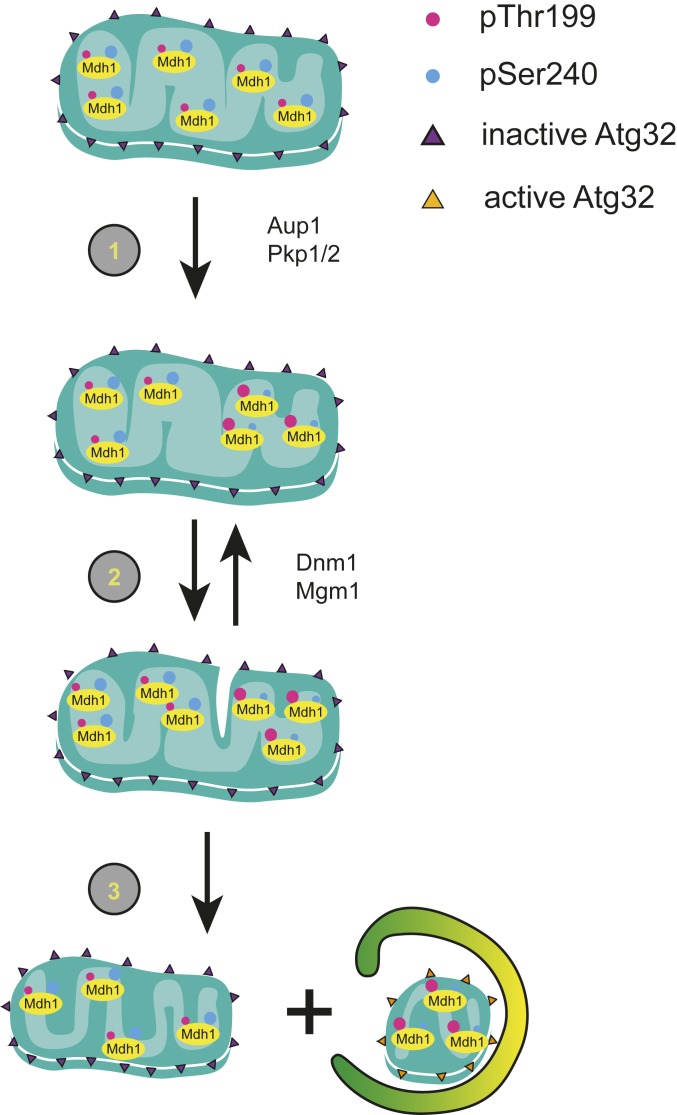
Fig. 7.
A model for the role of protein phosphorylation in regulating mitophagic selectivity. We suggest that phosphorylation of mitochondrial matrix proteins, such as Mdh1 can regulate their tendency to undergo mitophagy within the matrix. 1) Increased phosphorylation of T199 and decreased phosphorylation at S240 bring about a change in protein–protein interactions that leads to segregation of this species. 2) Multiple fusion and fission events lead to a “distillation”-like process that enriches this species in a single mitochondrial compartment, along with other protein species with similar properties. 3) A final fission event generates a mitochondrion sufficiently enriched in “degradation-bound” species. We hypothesize that the proteostatic load in this compartment generates a signal that activates the Atg32 receptor, possibly through Yme2-dependent clipping of the Atg32 C terminus, leading to engulfment of this compartment, with its enclosed protein components, by autophagic sequestering membranes.