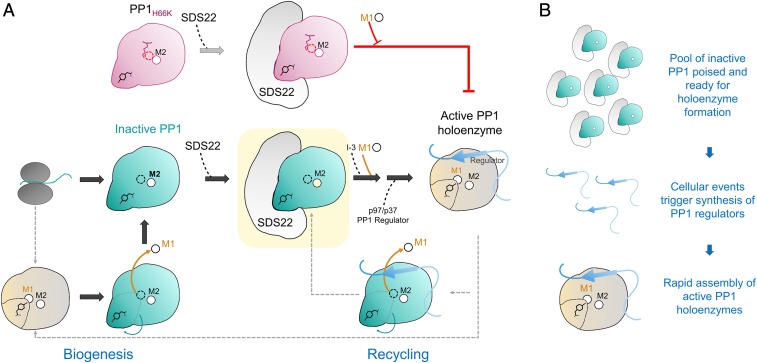
Fig. 6.
SDS22:PP1 provides a pool of inactive PP1 capable of rapidly assembling into active, functional PP1 holoenzymes. (A) Nascent PP1 folds and contains either 1 (M2) or 2 (M1 and M2) metals. Once folded, SDS22 then selectively binds and traps M1-metal-deficient PP1, stabilizing a unique conformation of PP1 that is catalytically inactive (all steps discovered in this study shown with large block arrows). The subsequent association of the SDS22:PP1 complex with I-3, metal (M1), and p37/p97, via unknown mechanisms, ultimately results in the dissociation of SDS22 from metal-loaded PP1 and, finally, PP1 holoenzyme formation. Critically, our data show that, if metal cannot be loaded into the M1 position of PP1 (i.e., as is case for the H66K variant, pink), PP1 holoenzyme formation does not occur and, instead, SDS22 remains constitutively bound to M1-metal-deficient PP1. Thus, M1-metal loading is strictly required for p37/p97 to effectively dissociate SDS22 from PP1. (B) SDS22 functions to bind and maintain PP1 in an inactive state, providing a pool of PP1 that is poised and ready for holoenzyme assembly (Top). As a result of cellular signaling events, distinct PP1 regulators are rapidly synthesized (Middle). These regulators (with M1, I-3, p97/p37; see A), via a mechanism not fully understood, then assemble into metal-loaded, active PP1 holoenzymes.