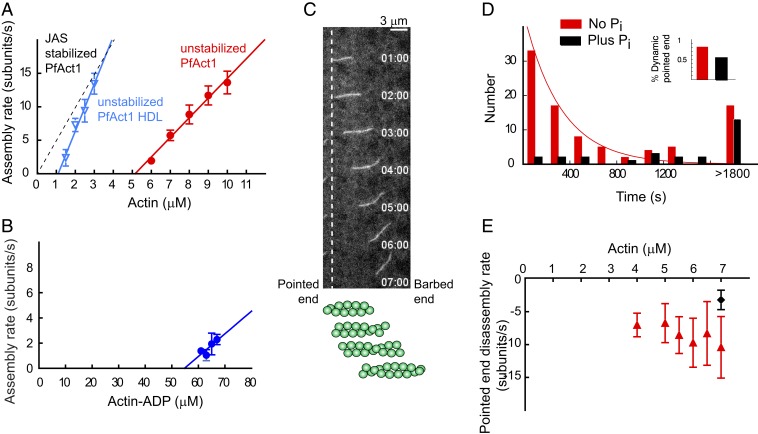
Fig. 3.
Assembly parameters of unstabilized PfAct1 filaments and the stabilizing effect of inorganic phosphate on pointed-end dynamics. (A) Polymerization rate at the barbed end as a function of actin concentration (red circles). Note the extremely high critical concentration of 5.0 µM actin (x-intercept), with an assembly rate constant of 3.0 subunits/µM·s (slope) and a disassembly rate constant of 15.0 subunits/s (y-intercept). The distinctly different polymerization curve in the presence of JAS (dashed black line) is shown for comparison. Compared with PfAct1, a mutant containing the D-loop from human actin (PfAct1-HDL; blue inverted triangles) decreased the critical concentration to 1.1 µM, with an assembly rate constant of 7.7 subunits/µM·s and a disassembly rate constant of 7.8 subunits/s. See Table 1 for average assembly and disassembly rate constants and critical concentration from multiple experiments. (B) Polymerization rate of actin-ADP at the barbed end as a function of actin-ADP concentration. See Materials and Methods for preparation of actin-ADP. The critical concentration (x-intercept) of ∼55 µM is 10-fold higher than for actin-ATP. See Table 1 for polymerization rate constants. (C) A treadmilling filament of PfAct1 near the critical concentration. Polymerization occurs at the barbed end simultaneously with depolymerization at the pointed end (vertical dashed line), without much change in overall length. The schematic below the data illustrates the flux of protomers. See also Movies S3 and S4. (D) The distribution of starting times at which the pointed end begins to depolymerize, fitted to an exponential with a decay constant of 0.003 s−1 (red bars). The last bin consists of filaments that remain intact after 30 min. Data from 5.5 to 7 µM G-actin were pooled; SI Appendix, Fig. S3 shows data at each actin concentration. Addition of 12.5 mM Pi (black bars) suppresses pointed-end depolymerization. Data from 2 experiments with 2 PfAct1 preparations (Movie S5). Inset shows that the percent of dynamic ends decreased from 81 to 53% in the presence of phosphate. (E) The pointed-end depolymerization rate is independent of actin concentration (7 to 10 s−1), and is reduced to approximately half (3.2 ± 1.5 s−1) in the presence of 12.5 mM Pi (black diamond) by favoring formation of bound ADP.Pi. Polymerization buffer: 10 mM imidazole, pH 7.5, 50 mM KCl, 2 mM MgCl2, 1 mM EGTA, 2.5 mM MgATP,10 mM DTT, 0.25% methylcellulose, 0.13 mg/mL glucose oxidase, 50 μg/mL catalase, and 3 mg/mL glucose.