Figure 1.
ALIX Interaction with PYL ABA Receptors.
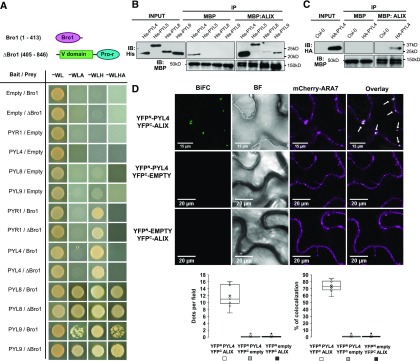
(A) The structure of ALIX showing the Bro1 domain (Bro1; amino acids 1 to 413) and the coiled coils plus the proline-rich region (∆Bro1; amino acids 405 to 846) are shown in the top panel. Y2H assays showing interaction of PYL ABA receptors with either Bro1 or ∆Bro1 domains. Transformed yeast cells were grown in synthetic drop-out (SD) medium lacking tryptophan and leucine (-WL) as a transformation control and in SD-WL media lacking adenine (–WLA), histidine (–WLH), or both (–WLHA) for interaction assays. Cotransformation with empty plasmids was used as negative control.
(B) Bacteria-purified PYL proteins fused to a 6x His tag (His-PYL) were pulled down on incubation with recombinant MBP-ALIX or MBP bound to amylose resin. Anti-His was used in immunoblots (IBs) to detect His-PYL fusions, and anti-MBP for MBP-ALIX and MBP.
(C) HA-PYL4 was pulled down on incubation of oeHA-PYL4 plant extracts with recombinant MBP-ALIX or MBP bound to amylose resin. Protein extracts from wild-type (Col-0) plants were used as negative control. Anti-HA was used to detect HA-PYL4 and anti-MBP for MBP-ALIX and MBP.
(D) In vivo interaction of ALIX with PYL4 assessed by BiFC. Confocal images were taken of N. benthamiana leaf epidermal cells expressing different BiFC construct combinations together with mCherry-ARA7, a MVB marker. Reconstitution of YFP fluorescence shows that ALIX and PYL4 constructs directly interact in mCherry-ARA7-labeled MVBs (i.e., punctae indicated by arrows in Overlay image). Bright-field (BF) images of the leaf areas are shown. Bars = 15 and 20 µm. The numbers of intracellular punctae per leaf section agroinfiltrated (y axis; dots per 50 × 50 µm field) as well as the percentage of colocalization of YFP-fluorescent and mCherry-ARA7 labeled vesicles (y axis; percentage of colocalization) with each construct combination (x axes) are quantified below the micrographs. Results were obtained from 10 fields from five biological replicates (two fields/replicate).