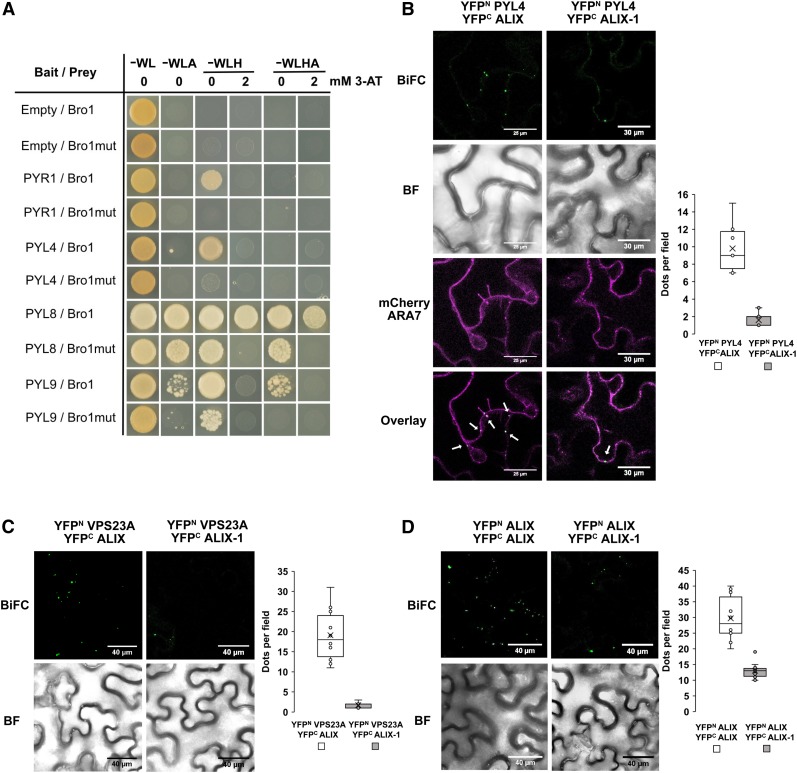
Figure 7.
ALIX-1 Mutant Protein Displays Reduced Interaction with ABA Receptors and the ESCRT Component VPS23A.
(A) Y2H assays for the interaction between the Bro1 domain of ALIX or a variant containing the alix-1 mutation (Bro1mut) and PYL ABA receptors. Transformed yeast cells were grown in synthetic drop-out medium lacking tryptophan and leucine (SD-WL) as a transformation control and in SD-WL media lacking adenine (−WLA), histidine (−WLH), or both (−WLHA) for interaction assays. 3-amino-1,2,4-triazole (3AT; 2mM) was added to better visualize positive interactions. Empty vectors were used as negative controls.
(B) to (D) BiFC assays showing impaired interaction of an ALIX version containing the alix-1 mutation (ALIX-1) with PYL4 (B), VPS23A (C), and wild-type ALIX (D). Bars = 30 µm (A) and 40 µm (C and D). Colocalization of YFP fluorescence by ALIX-PYL4 interaction with mCherry-ARA7-labeled MVBs is indicated by arrows in the Overlay image in (B). Bright-field (BF) images of the corresponding leaf areas are also shown in (B) to (D). The numbers of intracellular punctae per leaf section (y axis; dots per 50 × 50 µm field) agroinfiltrated with each construct combination (x axes) are quantified. Results were obtained from 10 fields from five biological replicates (2 fields/replicate).