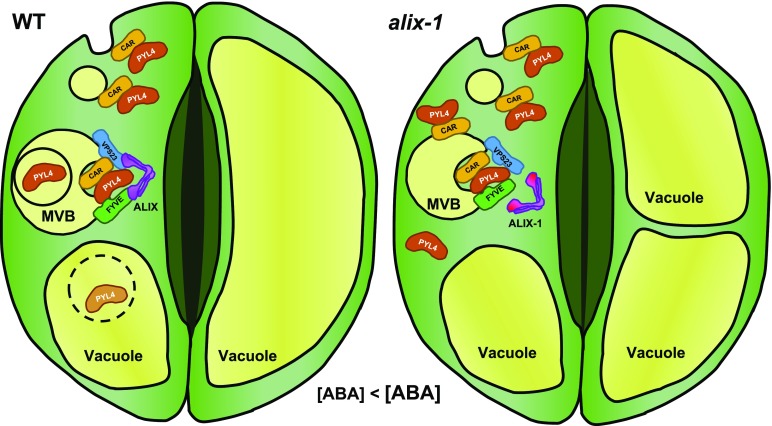
Figure 8.
Proposed Model for the Role of ALIX in the Endosomal Trafficking of ABA Receptors.
Soluble PYL4 proteins transiently bind to the plasma membrane via interaction with calcium binding CAR proteins undergoing subsequent endosomal trafficking for vacuolar degradation. MVB sorting of ABA receptors is mediated by the ESCRT machinery aided by ALIX. Vacuolar proteolysis of PYL4 is enhanced in the presence of ABA, likely as a desensitizing mechanism to this hormone. alix-1 mutation impairs the interaction with PYL4 and the ESCRT-I component VPS23A, hampering the internalization of PYL4 into intraluminal vesicles in MVBs. Thus, alix-1 leads to the accumulation of cytosolic and membrane-associated PYL4 that, together with increased ABA content in this mutant, increases sensitivity to ABA. Exacerbated response to ABA, together with vacuolar defects inherent to the alix-1 mutation, causes higher stomatal closure compared with wild-type plants.