Abstract
Background:
Next to aluminum salts, squalene nanoemulsions comprise the most widely employed class of adjuvants in approved vaccines. Despite their importance, the mechanisms of action of squalene nanoemulsions are not completely understood, nor are the structure/function requirements of the oil composition.
Purpose:
In this study, we build on previous work that compared the adjuvant properties of nanoemulsions made with different classes of oil structures to squalene nanoemulsion. Here, we introduce nanoemulsions made with polyprenols derived from species of the Pinaceae family as novel vaccine adjuvant compositions. In contrast with long-chain triglycerides that do not efficiently enhance an immune response, both polyprenols and squalene are comprised of multimeric isoprene units, which may represent an important structural property of oils in nanoemulsions with adjuvant properties.
Study Design:
Oils derived from species of the Pinaceae family were formulated in nanoemulsions, with or without a synthetic Toll-like receptor 4 (TLR4) ligand, and characterized regarding physicochemical and biological activity properties in comparison to squalene nanoemulsions.
Methods:
Oils were extracted from species of the Pinaceae family and used to prepare oil-in-water nanoemulsions by microfluidization. Emulsion droplet diameter stability was characterized by dynamic light scattering. Nanoemulsions were evaluated for in vitro biological activity using human whole blood, and in vivo biological activity in mouse, pig, and ferret models when combined with pandemic influenza vaccine antigens.
Results:
Nanoemulsions comprised of Pinaceae-derived polyprenol oils demonstrated long-term physical stability, stimulated cytokine production from human cells in vitro, and promoted antigen-specific immune responses in various animal models, particularly when formulated with the TLR4 ligand glucopyranosyl lipid adjuvant (GLA).
Conclusion:
Pinaceae-derived nanoemulsions are compatible with inclusion of a synthetic TLR4 ligand and promote antigen-specific immune responses to pandemic influenza antigens in mouse, pig, and ferret models.
Keywords: conifers, polyprenols, squalene, vaccine adjuvant, influenza, nanoemulsion
Graphical Abstract
Introduction
Squalene-based oil-in-water (o/w) emulsions are among the few adjuvant formulations in vaccines approved for human use, with some 200,000,000 doses administered to date (Fox and Haensler, 2013). A wealth of preclinical and clinical data is available regarding the safety and immune potentiating effects of squalene emulsions such as MF59® (O'Hagan et al., 2013). Using recombinant or inactivated H5N1 antigens, we and others have shown that squalene-based emulsions provide significant antibody and cell-mediated immunity enhancements and antigen dose sparing potential in preclinical and clinical tests (Fox et al., 2013; Fox and Haensler, 2013). Despite the recognized importance and broad use of squalene emulsions, the specific mechanisms of action responsible for their adjuvant activity remain unclear, even after several studies that have revealed potential contributors, such as enhanced immune cell recruitment and antigen uptake (O'Hagan et al., 2012).
In current practice, the squalene that is used in vaccine adjuvant formulations is extracted from shark liver, where it is found in high concentrations (Popa et al., 2015; Wetherbee and Nichols, 2000). While vaccine manufacturers are not the primary market for shark-extracted squalene (Brito et al., 2011), reliance upon animals as a source for raw materials raises questions about sustainability. In this context, emulsions made from plant-derived squalene have been reported to demonstrate comparable stability and adjuvant activity as shark-derived squalene (Brito et al., 2011; Fox et al., 2008). Moreover, emulsions containing synthetic polyisoprene polymers demonstrated in vitro immunostimulatory activity on human cells (Adlington et al., 2016). In contrast, emulsions containing different classes of biocompatible oils such as long chain triglycerides, medium chain triglycerides, or perfluorocarbons, demonstrated little or no adjuvant activity when employed with influenza or other antigens (Fox et al., 2011; Fox et al., 2013; Orr et al., 2013). The reasons for the significantly enhanced adjuvant properties of squalene emulsions compared to emulsions made with other metabolizable oils are not clear.
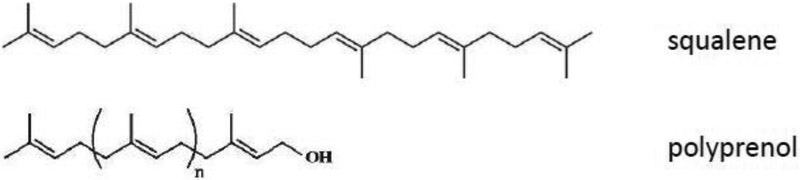
Oils derived from conifer needles offer unique compositional characteristics. Whereas other vegetable oils are often triglyceride-based, conifer needle-derived oil is mainly comprised of polyprenols (Narovlyansky et al., 2018). The polyprenol-containing Ropren® is a pharmaceutical product approved for use in Russia to treat liver disease, and has demonstrated other therapeutic uses as well (Fedotova et al., 2012). Polyprenols, like squalene, are comprised of multimerized isoprenes (Fig. 1). We sought to determine how emulsions made from conifer-derived oils compared with squalene emulsions regarding adjuvant activity when combined with an inactivated influenza vaccine. To our knowledge, this is the first evaluation of these polyprenols for vaccine adjuvant applications.
Figure 1. Chemical structures of squalene and polyprenol.
Materials and methods
Production and characterization of polyprenols
The methodology and chemical characterization of pharmaceutical-grade long-chain isoprenoid alcohols (polyprenols) extracted from the green verdure of Picea abies (L.) H. Karst (Pinaceae) and produced at pharmaceutical concentrations of not less than 95% purity has been previously described (Fedotova et al., 2012; Roschin and Soultanov, 2003). The extraction methods employed in the present work were detailed previously (Roschin and Soultanov, 2003). The finished form of this substance is registered in Russia as Ropren® (Prenolica Limited, Australia, formerly known as Solagran Limited) and contains 25% pharmaceutical-grade polyprenols from P. abies and 75% vegetable oil. The functional activity of polyprenols from P. abies has been characterised and extensively studied (Fedotova et al., 2012; Fedotova et al., 2016; Soultanov et al., 2017; Soultanov, 2016). For this study, the same established methodology for extracting pharmaceutical-grade polyprenols was used to produce batches of polyprenols from three conifer species, P. abies, Abies sibirica Ledeb. (Pinaceae) and Pinus sibirica (Pinaceae).
Emulsion manufacture and characterization
Emulsion raw materials included polyprenols prepared as described above, glucopyranosyl lipid adjuvant [GLA] (Avanti Polar Lipids), squalene (Sigma-Aldrich), dimyristoyl phosphatidylcholine (Lipoid LLC), poloxamer 188, glycerol, and monobasic and dibasic ammonium phosphate salts (all from Spectrum Chemical). Emulsions were manufactured by microfluidization at 10% or 4% v/v oil essentially as previously described (Misquith et al., 2014), and diluted to 2% oil upon mixing with antigen for injection. Emulsion characterization was performed as described previously (Misquith et al., 2014) using dynamic light scattering to assess particle diameter and polydispersity, visual appearance, and HPLC with charged aerosol detection for GLA quantitation. GLA quantitation was only possible in squalene emulsions since the co-elution of polyprenols with GLA prevented quantitation in polyprenol emulsions.
In vitro human whole blood stimulation assay
Informed consent was obtained from eight subjects and the study was approved by Western IRB, Seattle, WA. . The squalene emulsions were diluted from 10% oil to 4% oil with saline to be equivalent to the 4% oil polyprenol emulsions. Two dilutions, 1:4 and 1:10, of each formulation in saline were prepared to obtain 1% and 0.4% oil final concentration. For a 1:4 dilution, 50 μl formulation was added to 150 μl of heparinized whole blood using 96-well round bottom tissue culture plates, in duplicate. For a 1:10 dilution, 50 μl formulation was added to 450 μl heparinized whole blood using 48-well flat bottom tissue culture plates. The plates were incubated at 37 °C and 5% CO2 for 24 h. After incubation, 150 μl extractions of the plasma supernatant from each well were aspirated and assayed for IL-lβ, IL-1RA, IL-6, IL-8, IL-10, IL-12p40, TNF, IFNγ, IP-10, MCP-1, Mip-lα, Mip-lβ, GRO, ENA-78, and G-CSF using a custom Luminex-based multiplex immunoassay kit (ProCartaPlex Assay Kit, eBioscience, San Diego, CA). For heat mapping, the data were scaled by the sum of the values in each row.
Animal studies
All murine immunogenicity studies were carried out in the IDRI animal care facility (Seattle, WA) under specific pathogen-free conditions, and in accordance with previously approved techniques. All animal experiments and protocols used in this study were specifically approved by IDRI’s Institutional Animal Care and Use Committee (IACUC). The pig and ferret studies were carried out at Colorado State University (CSU) in accordance with established experimental procedures, and were specifically approved prior to initiation by the CSU IACUC.
Results from control groups (saline, antigen alone, antigen + squalene emulsion) in some of the animal studies were previously reported as part of a concurrent study (Van Hoeven et al., 2017). Pseudotype microneutralization (MN) assay, hemagglutination inhibition (HAI) assay, serum antibody ELISAs, and virus titer plaque assay were performed as previously described (Van Hoeven et al., 2017).
For murine studies groups of 6–8 week old female C57Bl/6 mice were immunized with split inactivated H5N1 (Sanofi Pastuer, sourced from the national pandemic stockpile) or recombinant H5 protein (VN1203 strain, Protein Sciences Corp.) combined with adjuvant emulsions as indicated. Mice were immunized intramuscularly in a total volume of 100 μl split evenly between both legs. H5N1 dose was 0.5 μg and GLA dose was 5 μg. For antigen ELISAs, each time point represents 4-10 mice in each group. For ELISpot assays, each timepoint represents 5 mice per group.
Yorkshire pigs were obtained from a commercial producer at 12-14 weeks of age. They were immunized twice, 3 weeks apart, by intramuscular injection of 1 ml into a rear leg. Serum samples were collected at the time of, and 21, 28, and 42 days after primary immunization for serologic testing. H5N1 dose was 10 μg and GFA dose was 5 μg. For antigen ELISAs, each time point represents 4-5 pigs.
Male Fitch ferrets (Mustela putoris fero, Triple F Farms, Sayre PA) were used for all immunization and challenge studies. Immunization of ferrets was carried out by injection of 250 μl into the quadriceps muscle. All animals received 0.5 μg of a split A/Vietnam/1203/04 H5N1 vaccine (Sanofi Pasteur) sourced from the national pandemic stockpile. Serum samples were collected 21 days post-immunization to determine antibody titers and the ferrets were challenged at that time by intranasal instillation of 1 × 105–5 × 105 PFU of A/Vietnam/1203/2004 virus in a volume of 500 μl (250 μl/nare). Following infection, animals were observed and weighed daily to monitor virus-induced morbidity, and nasal washes were collected under ketamine-xylazine sedation on days 1, 3, 5, 7, and 9 post-challenge for virus titration. Any animal displaying severe clinical signs or losing >25% of pre-challenge weight was euthanized. Each group contained 3-4 ferrets.
Statistical analysis
Statistical analysis was performed using GraphPad Prism v7.04. Antibody titer data were log-transformed prior to statistical analysis. Normal distributions were assumed for groups with small numbers of animals (≤5) since the D’Agostino & Pearson normality test is only suitable for larger numbers per group. For groups with ≥10 animals, the D’Agostino & Pearson normality test was performed. If the data failed the normality test, the non-parametric Kruskal-Wallis test was performed with Dunn’s correction for multiple comparisons. For data demonstrating normal distribution or with assumed normal distribution, one-way ANOVA was performed with Tukey’s correction for multiple comparisons. If the condition of equality of variance between groups was not met as determined by the Brown-Forsythe test, then the data were analyzed by Welch’s ANOVA with Games-HoweH’s correction for multiple comparisons. Antibody ELISAs were fit to a variable slope sigmoidal dose-response curve. If the curve fit did not converge or did not drop below the cutoff value, the titer was assigned as the highest value that was above the cutoff. An arbitrary titer of 1 was assigned if the measured values were below the cutoff value and no curve fit was obtained.
Results
Polyprenol composition of P. abies, A. sibirica, and P. sibirica
Polyprenols were extracted from the green verdure of P. abies (Norway spruce), A. sibirica (Siberian fur), or P. sibirica (Siberian pine) and were purified to >90% polyprenol content (Table 1). All polyprenol materials passed established specifications for appearance, color, odor, solubility, density, refractive index, purity, residual solvents, heavy metals, and microbiological analysis. Ropren® consisted of 25% w/w polyprenols from P. abies in vegetable oil. The molecular polyprenol content in undiluted samples from each source species as determined by HPLC analysis is shown in Table 2. For each polyprenol species, HPLC chromatograms resolving individual polyprenol homologues as well as total polyprenol content are located in Supplementary Information. Various differences in appearance and composition between the polyprenols from different sources are notable. Thus, color of the oils appeared to correlate with purity, with a light yellow appearance for the P. abies oil (96.2% purity), yellow appearance for the A. sibirica oil (95.3% purity), and yellow-orange appearance for the P. sibirica oil (90.8% purity). Polyprenols from P. abies are represented by homologues from C50 to C100. The homologue with the maximum quantity is C75 (34.8%). The main group of homologues from C70 to C85 represents 89.7% of the total mass. Polyprenols from A sibirica are represented by homologues from C50 to C95. The homologues with the maximum quantity are C80 (32.5%) and C85 (38.2%). The main group of homologues from C75 to C90 represents 90.6% of the total mass. Polyprenols from P. sibirica are represented by homologues from C50 to C100. The homologues with the maximum quantity are the homologues of C80 (25.9%) and C85 (27.5%). The main group of homologues from C75 to C90 is 74.4% of the total mass. We also note in P. sibirica the presence of dolichols from C75 to C100 with a total amount of 4.0%. Thus, polyprenols from A. sibirica and P. sibirica have a higher molecular weight than polyprenols from P. abies. The polyprenols were stored at 2-8°C until used.
Table 1.
Polyprenol material properties
| Species | Purity (%) |
Density (g/cm3) |
color | Refractive index |
|---|---|---|---|---|
| P. abies | 96.2 | 0.899 | light yellow | 1.5131 |
| A. sibirica | 95.3 | 0.897 | yellow | 1.5118 |
| P. sibirica | 90.8 | 0.901 | yellow-orange | 1.5126 |
Notes: The material from P. sibinca includes ~4% dolichols. Dolichols were not detected in the material from P. abies or A. sibirica. The standard deviation for the relative percentage of polyprenols is determined by the metrological characteristics of the equipment used for liquid chromatography and amounts to 1% of the concentration value of polyprenols from each species.
Table 2.
Polyprenol molecular compositions of undiluted samples from each source species
| No. of isoprene units |
Carbon number |
MW | common name | Relative % | ||
|---|---|---|---|---|---|---|
| P. abies |
A. sibirica |
P. sibirica |
||||
| 10 | C50 | 699.2 | decaprenol | 0.4 | 0.3 | 0.4 |
| 11 | C55 | 767.3 | undecaprenol | 0.5 | 0.1 | 0.5 |
| 12 | C60 | 835.4 | dodecaprenol | 0.9 | 0.2 | 0.2 |
| 13 | C65 | 903.6 | tridecaprenol | 2.5 | 0.3 | 0.9 |
| 14 | C70 | 971.7 | tetradecaprenol | 15.6 | 1.1 | 2.8 |
| 15 | C75 | 1039.8 | pentadecaprenol | 34.8 | 8.0 | 10.7 |
| 16 | C80 | 1107.9 | hexadecaprenol | 27.8 | 32.5 | 25.9 |
| 17 | C85 | 1176.0 | heptadecaprenol | 10.5 | 38.2 | 27.5 |
| 18 | C90 | 1244.2 | octadecaprenol | 3.2 | 11.9 | 10.3 |
| 19 | C95 | 1312.3 | nonadecaprenol | 0.8 | 0.7 | 3.9 |
| 20 | C100 | 1380.4 | eicosaprenol | 0.1 | 0.0 | 3.0 |
Nanoemulsion production and stability
Oil-in-water nanoemulsions consisting of oil (polyprenols, squalene, or grapeseed), dimyristoyl phosphatidylcholine (DMPC), poloxamer 188, glycerol, and ammonium phosphate buffer (pH 5.8) were manufactured using high pressure homogenization essentially as previously described (Misquith et al., 2014). Selected nanoemulsions were also manufactured to contain a synthetic Toll-like receptor 4 (TLR4) ligand, GLA. Squalene nanoemulsions and long-chain triglyceride (grapeseed) nanoemulsions were manufactured for comparison to the polyprenol emulsions. All emulsions were assessed for visual appearance and particle size by dynamic light scattering within approximately one month following manufacture, with samples stored at 2-8°C (Table 3). Average particle size was 80-121 nm for all emulsions and polydispersity index values were ≤0.13, indicating generally monodisperse nanoemulsions. No major difference in emulsion physical characteristics was apparent between emulsions made with the different sources of polyprenols, squalene, or grapeseed oil. Replicate batches of selected emulsions demonstrated acceptable reproducibility (Table 3). Remarkable long-term physical stability was demonstrated by the polyprenol nanoemulsions at 2-8°C and even at 37°C for selected compositions (Supplementary Table 1).
Table 3.
Nanoemulsion physical characteristics.
| Description | Visual appearance | Particle diameter (Z-ave, nm) |
Size polydispersity index (PdI) |
|---|---|---|---|
| P. abies nanoemulsion | Homogeneous, milky-white | 114 +/− 1 | 0.13 +/− 0.01 |
| A. sibirica nanoemulsion | Homogeneous, milky-white | 104 +/− 1 | 0.06 +/− 0.02 |
| Homogeneous, milky-white | 98 +/− 1 | 0.05 +/− 0.01 | |
| P. sibirica nanoemulsion | Homogeneous, yellow | 103 +/− 1 | 0.06 +/− 0.03 |
| Ropren® nanoemulsion | Homogeneous, milky-white | 94 +/− 0 | 0.05 +/− 0.02 |
| Squalene nanoemulsion** | Homogeneous, milky-white | 121 +/− 1 | 0.04 +/− 0.04 |
| Homogeneous, milky-white | 87 +/− 2 | 0.05 +/− 0.02 | |
| Grapeseed nanoemulsion | Homogeneous, milky-white | 91 +/− 1 | 0.06 +/− 0.02 |
| A. sibirica nanoemulsion + GLA | Homogeneous, milky-white | 106 +/− 1 | 0.08 +/− 0.02 |
| Squalene nanoemulsion + GLA** | Homogeneous, milky-white | 80 +/− 0 | 0.07 +/− 0.02 |
| Homogeneous, milky-white | 84 +/− 1 | 0.05 +/− 0.02 |
Manufactured at 10% oil concentration; all other nanoemulsions manufactured at 4% oil concentration.
In vitro biological activity of nanoemulsions
The manufactured nanoemulsions were evaluated for their ability to stimulate human whole blood to produce cytokines according to a previously described approach (Adlington et al., 2016). Consistent with previous experience, squalene nanoemulsion upregulated production of multiple cytokines (Fig. 2a). Nanoemulsions comprised of polyprenols from A. sibirica or P. sibirica elicited limited increases in selected cytokines, whereas nanoemulsions containing polyprenols from P. abies (including Ropren®) caused little or no change in cytokine production. Addition of GLA to selected nanoemulsions induced production of multiple cytokines (Fig. 2b), as anticipated based on previous reports (Carter et al., 2016; Misquith et al., 2014). Nevertheless, the squalene + GLA composition elicited higher levels of cytokines compared to polyprenol + GLA compositions. Overall, squalene nanoemulsion compositions demonstrated greater in vitro cytokine stimulation activity than polyprenol-based nanoemulsions.
Figure 2. Effect of squalene-based nanoemulsions and polyprenol-based nanoemulsions on cytokine production from stimulated human whole blood.
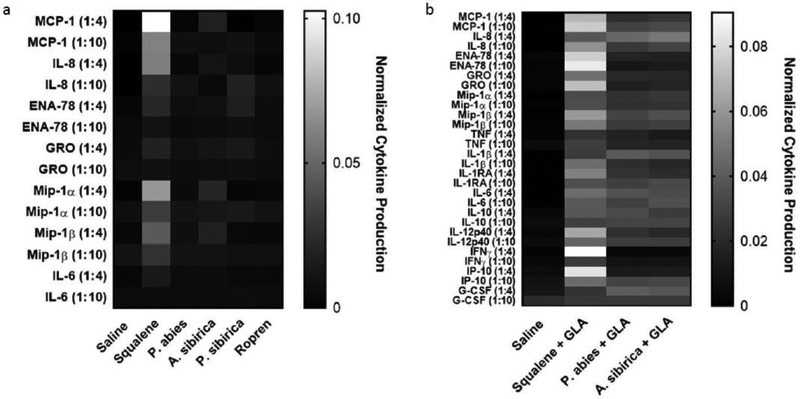
(a) Cytokines stimulated by nanoemulsions. (b) Cytokines stimulated by nanoemulsions + GLA. Panels a) and b) derive from the same experiment with the same saline control, but are separated for easier visualization.
In vivo biological activity of nanoemulsions combined with influenza vaccine
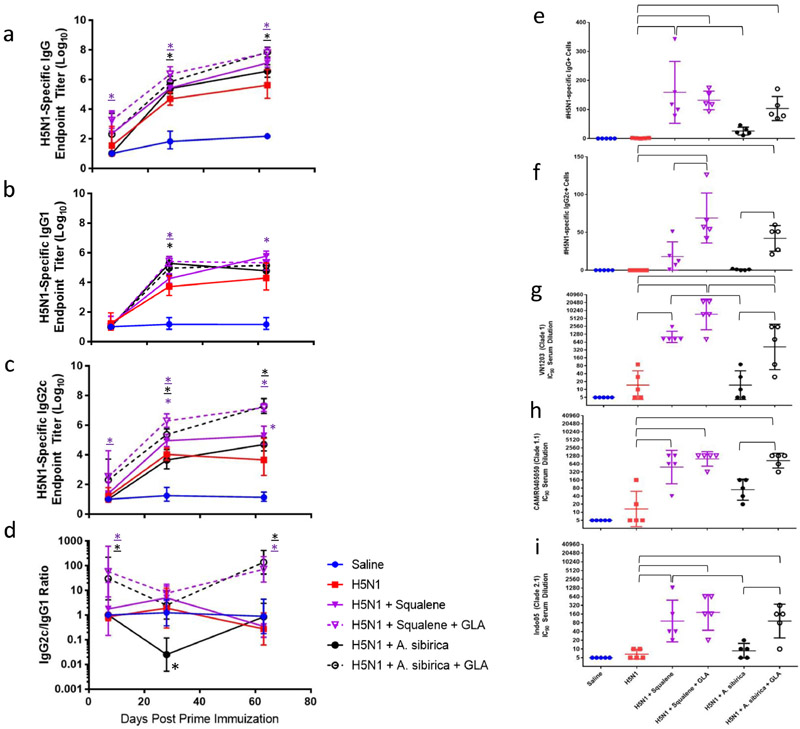
To evaluate nanoemulsion in vivo adjuvant activity, C57BL/6 mice were immunized twice by the intramuscular route with split inactivated H5N1 vaccine immediately after mixing the antigen with the nanoemulsion compositions, with four weeks between each immunization. Antigen-specific serum IgG, IgG1, and IgG2c antibodies collected 7, 21, 27, and 42 days after the prime immunization indicated enhanced antibody production in mice receiving vaccine compositions containing squalene, A. sibirica, or P. sibirica compared to antigen alone (Fig. 3a-c). In contrast, vaccine compositions containing P. abies or long chain triglycerides (Grapeseed, Ropren®) led to equivalent or reduced antibody titers compared to antigen alone. Statistically significant increase in long-lived antibody-secreting plasma cells was only achieved by the vaccine composition containing squalene, although a trend for increased response attributable to polyprenol (P. abies, A. sibirica, or P. sibirica) inclusion was evident (Fig. 3e-f). IgG subtype ratio analysis indicated that all adjuvant compositions generally induced a Th2-type response (Fig. 3d). Taken together, the A. sibirica and P. sibirica nanoemulsions demonstrated the greatest potential to enhance antibody responses among the polyprenol-based emulsions tested, but were not as potent as squalene nanoemulsion. Although no difference in adjuvant activity was apparent between A. sibirica and P. sibirica nanoemulsions, the former was available at higher purity (Table 1) and was selected for subsequent experiments focused on further optimization of the polyprenol-based adjuvant composition.
Figure 3. Effect of nanoemulsion oil composition on antigen-specific antibody response levels in immunized mice.
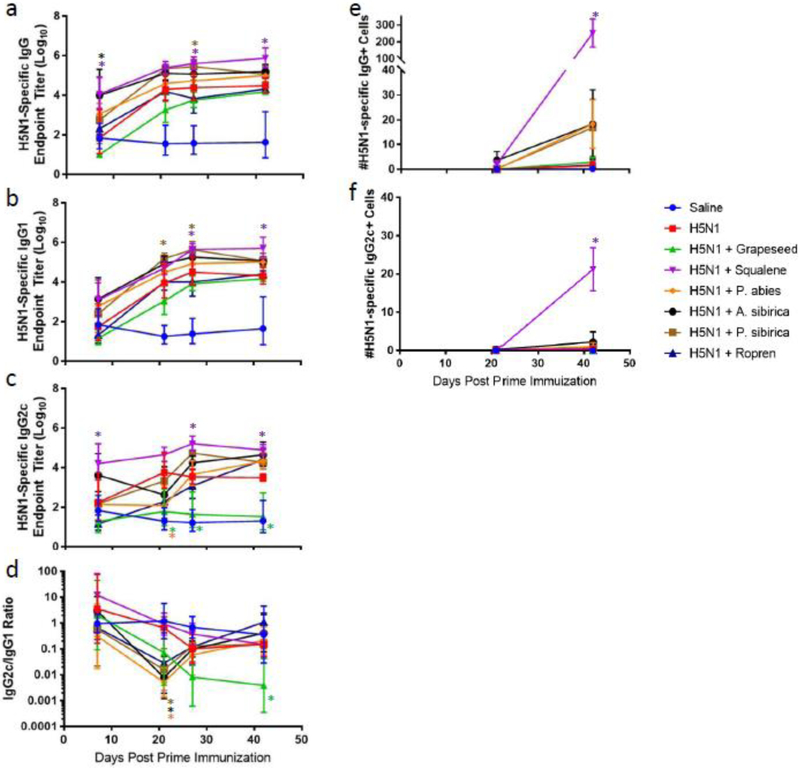
(a) Geometric mean (+/− geometric s.d.) of antigen-specific total IgG endpoint titers as measured by ELISA. (b) Geometric mean (+/− geometric s.d.) of antigen-specific IgG1 endpoint titers as measured by ELISA. (c) Geometric mean (+/− geometric s.d.) of antigen-specific IgG2c endpoint titers as measured by ELISA. (d) Geometric mean (+/− geometric s.d.) of IgG2c/IgG1 ratios. (e) Arithmetic mean (+/− s.d.) of antigen-specific IgG bone marrow long-lived antibody secreting cells as measured by ELISpot assay. (f) Arithmetic mean (+/− s.d.) of antigen-specific IgG2c bone marrow long-lived antibody secreting cells as measured by ELISpot assay. *p<0.05 vs H5N1 (color of asterisk represents group).
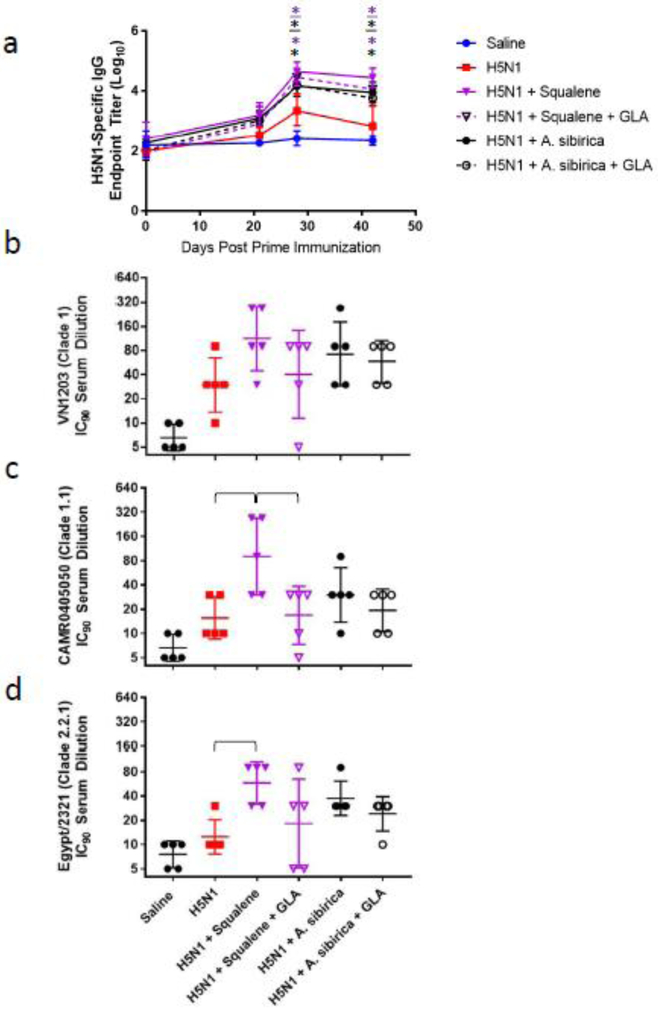
To determine the effect of inclusion of a synthetic TLR4 ligand, GLA was formulated in squalene nanoemulsion or A. sibirica nanoemulsion (Table 3). The adjuvant formulations were combined with split inactivated H5N1 vaccine, and C57BL/6 mice received two intramuscular immunizations (three weeks apart). As expected, the adjuvanted groups appeared to increase antigen-specific serum antibody responses (Fig. 4a-c). The Th1-biasing effect of GLA inclusion in the squalene or A. sibirica nanoemulsions was clearly evident in the enhanced serum IgG2c, IgG2c/IgG1 ratio, and long-lived plasma cell IgG2c responses (Fig. 4c-f). With regards to functional antibody response, the squalene nanoemulsion-induced neutralization titers were significantly higher than the titers elicited by A. sibirica nanoemulsion (Fig. 4g-i). Interestingly, inclusion of GLA significantly enhanced neutralizing titers compared to A. sibirica nanoemulsion alone, whereas no significant enhancement was detected when GLA was added to squalene nanoemulsion compared to squalene nanoemulsion alone.
Figure 4. Effect of inclusion of a synthetic TLR4 ligand on antibody immune response qualityfor squalene and polyprenol nanoemulsions in immunized mice.
(a) Geometric mean (+/− geometric s.d.) of antigen-specific total IgG endpoint titers as measured by ELISA. (b) Geometric mean (+/− geometric s.d.) of antigen-specific IgG1 endpoint titers as measured by ELISA. (c) Geometric mean (+/− geometric s.d.) of antigen-specific IgG2c endpoint titers as measured by ELISA. (d) Geometric mean (+/− geometric s.d.) of IgG2c/IgG1 ratios. (e) Arithmetic mean (+/− s.d.) of antigen-specific IgG bone marrow long-lived antibody secreting cells as measured by ELISpot assay 63 days after prime immunization. (f) Arithmetic mean (+/− s.d.) Antigen-specific IgG2c bone marrow long-lived antibody secreting cells as measured by ELISpot assay 63 days after prime immunization. (g-i) Geometric mean (+/− geometric s.d.) of virus neutralizing antibodies to homologous or heterologous strains measured 35 days after prime immunization using a retrovirus pseudotype neutralization assay. *p<0.05 vs H5N1 (color of asterisk represents group, underlined asterisk represents inclusion of GLA).
In the above experiments employing split-inactivated H5N1 antigen, squalene nanoemulsion demonstrated somewhat greater magnitude of adjuvant activity than A. sibirica nanoemulsion in selected readouts (e.g. Fig. 3c-f and Fig. 4e,g,i) involving small numbers of mice. However, variability in this trend was evident in some experiments involving more mice or a different antigen. Thus, functional antibody responses (MN and HAI) in groups of 20-25 mice immunized twice with split-inactivated H5N1 antigen indicated equivalent or enhanced responses with A. sibirica nanoemulsion compared to the squalene nanoemulsion comparators (Fig. 5). Moreover, when combined with a recombinant H5 antigen, functional antibody and long-lived plasma cell responses generated by A. sibirica nanoemulsion (with or without GLA) were nearly identical to the squalene nanoemulsion comparators (Supplementary Fig. 1). Taken together, A. sibirica nanoemulsion containing GLA demonstrates comparable antibody adjuvant activity as squalene nanoemulsion with GLA in immunized mice, whereas performance of the A. sibirica nanoemulsion without GLA in comparison to squalene nanoemulsion was more variable.
Figure 5. Functional antibody responses to homologous and heterologous strains in mice immunized with squalene or polyprenol nanoemulsions.
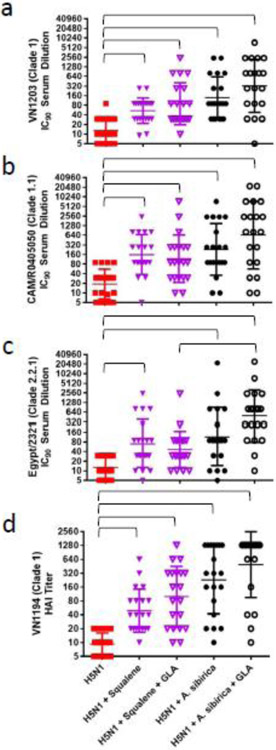
(a-c) Geometric mean (+/− geometric s.d.) of virus neutralizing antibodies to homologous or heterologous strains measured 42 days after prime immunization using a retrovirus pseudotype neutralization assay. (d) Geometric mean (+/− geometric s.d.) of hemagglutination inhibition titers to the homologous strain measured Day 42 after prime immunization.
To determine the adjuvant activity of nanoemulsions in different animal models, we evaluated the effect of nanoemulsion adjuvant formulations on total IgG and functional antibody responses in pigs and protection from influenza challenge in ferrets. In contrast to mice, the pig immunome is considered to more closely approximate that of humans (Overgaard et al., 2015). Furthermore, the ferret is well established as the most relevant influenza challenge model (Belser et al., 2011; Oh and Hurt, 2016). Moreover, compared to mice, the injection volumes and doses for pigs and ferrets may more closely approximate those used for humans.
Pigs (4-5/group) were immunized twice intramuscularly, three weeks apart, with split inactivated H5N1 compositions combined with squalene or A. sibirica nanoemulsion formulations. They were monitored for antigen-specific total serum IgG as well as microneutralization titers against homologous and heterologous strains (Fig. 6). Both squalene and A. sibirica nanoemulsion significantly increased IgG titers at Day 28 and Day 42 post-prime immunization compared to antigen alone (Fig. 6a). Regarding microneutralization titers, no formulation generated statistically significant response to the homologous strain (Fig. 6b), whereas squalene nanoemulsion elicited higher heterologous response than antigen alone (Fig. 6c-d). Interestingly, no benefit of TLR4 agonist (GLA) inclusion was evident for either squalene or A. sibirica nanoemulsions. Moreover, squalene nanoemulsion without GLA elicited higher microneutralization titers against the Clade 1.1 heterologous strain compared to squalene nanoemulsion with GLA (Fig. 6c).
Figure 6. Antigen-specific IgG and functional antibody responses in pigs immunized with squalene or polyprenol nanoemulsions.
(a) Geometric mean (+/− geometric s.d.) of antigen-specific total IgG endpoint titers as measured by ELISA. (b-d) Geometric mean (+/− geometric s.d.) of virus neutralizing antibodies to homologous or heterologous strains measured 42 days after prime immunization using a retrovirus pseudotype neutralization assay. *p<0.05 vs H5N1 (color of asterisk represents group, underlined asterisk represents inclusion of GLA).
Ferrets (3-4/group) were immunized once intramuscularly with split inactivated H5N1 compositions combined with squalene or A. sibirica nanoemulsion formulations. Twenty-one days following the immunization, ferrets were challenged intranasally with 106 PFU of A/VN/1203/04. The animals were monitored for 14 days post-challenge for survival, body weight, clinical score, temperature and nasal virus titer. Prior to challenge, ferret sera were collected and assayed for homologous and heterologous microneutralization titers. Due to the small number of animals, statistical significance was not generally achieved. However, some trends were consistent: Formulations containing GLA demonstrated the highest survival rates and homologous microneutralization titers as well as the lowest virus titers in nasal washes and the least change in body weight. In the absence of GLA, A. sibirica nanoemulsion generally performed comparably, if not better, than the squalene nanoemulsion.
Discussion
The vaccine adjuvant activity associated with oil-in-water emulsions is generally not well understood. The results presented here build on previous findings by introducing polyprenol oils that - when formulated in nanoemulsions with pandemic influenza antigens - enhance antigen-specific antibody responses; particularly when formulated with the TLR4 agonist GLA. These findings confirm previous reports that long chain triglyceride-based nanoemulsions are not effective vaccine adjuvants (Fox et al., 2011), and may even suppress selected responses compared to antigen alone (Fig. 3c). However, nanoemulsions based on polyprenols derived from A. sibirica or P. sibirica showed a tendency to enhance cytokine production from stimulated human whole blood and promoted antigen-specific serum antibodies in immunized mice. Interestingly, nanoemulsions containing polyprenols from P. abies (or a mixture of P. abies with vegetable oil) did not appear to increase immune response to the same extent as nanoemulsions made with P. sibirica or A. sibirica. Thus, not only do polyprenol-based nanoemulsions demonstrate greater adjuvant activity than long-chain triglyceride emulsions, but nanoemulsions containing polyprenols derived from different conifer sources also show slightly differential adjuvant performance. In this regard, it is interesting to note that the overall chemical composition of the A. sibirica and P. sibirica polyprenols is skewed towards longer chain lengths compared to the P. abies polyprenols (see Table 2 and Results section above). Thus, whereas oil derived from P. abies was comprised of ~20% of shorter chain polyprenols (≤70 carbons [tetradecaprenol, tridecaprenol, dodecaprenol, undecaprenol and decaprenol]), oils from A. sibirica and P. sibirica contained <5% of these same short-chain polyprenols. Likewise, oils derived from A. sibirica and P. sibirica contained 44-51% of long-chain polyprenols (≥85 carbons; [heptadecaprenol, octadecaprenol, nonadecaprenol, and eicosaprenol]) compared to <15% of the same long-chain polyprenols contained in oil derived from P. abies. Other differences did not appear to have substantial effects on biological activity. For instance, the presence of 4% dolichols in the oil derived from P. sibirica as well as the relatively high amount of nondecaprenol and eicosaprenol did not appear to differentiate it from the oil derived from A. sibirica in terms of biological activity or emulsion stability. All three polyprenol oils contained a similar proportion of the 80-carbon hexadecaprenol (26-33%).
Thus, differences in adjuvant activity between species may be due to the fact that polyprenols from A. sibirica and P. sibirica have a higher molecular weight than polyprenols from P. abies. It is also possible that physical characteristics of the emulsions would impact their biological activity; however, as average droplet diameters were similar between the various emulsions, it is possible that oil composition is the more relevant attribute. However, further investigation is needed to identify the impact of specific differences in polyprenol composition on adjuvant activity.
The performance of the various adjuvant formulations across different animal species was notable. Whereas in mice and ferrets the inclusion of GLA appeared to enhance functional antibody, IgG2c response, and/or protection from challenge, no benefits of GLA inclusion were apparent in the pig model. This highlights the limitations of animal models regarding evaluation of novel adjuvant compositions. While selected readouts in the mouse model indicated somewhat lower potency of polyprenol nanoemulsions compared to the squalene nanoemulsion, the overall performance of the A. sibirica polyprenol nanoemulsions (with or without GLA), taking into account results from the three animal models, approached that of the squalene nanoemulsion counterparts.
In related work, it was shown that nanoemulsions made with chemically synthesized polyisoprenes also indicate promising immunostimulatory activity (Adlington et al., 2016); the polyisoprenes in this case were linear oligomers synthesized using a catalytic chain transfer polymerization approach which yielded distributions of molecules with molecular weights of ~2000-3000 g/mol having the same 1,4 addition form of the isoprene monomer as is found in natural squalene. Thus, the work in Adlington et al. combined with the present results appear to indicate that polyisoprene-based structures of up to ~7x-higher molecular weights than squalene maintain adjuvant activity. Moreover, the results reported in the present work indicate that a terminal alcohol group (i.e. polyprenols) does not have a detrimental effect on adjuvant properties of polyisoprenoids. Given that squalene, synthetic polyisoprenes, and Pinaceae-derived polyprenols share the structural motif of polymeric isoprene units, additional study on the structure-function relationship of such oils is warranted as it could help inform the mechanistic understanding of how oil-in-water emulsions function as adjuvants.
Conclusions
The ability to use plant-based isoprenoid substances in nanoemulsion adjuvants could improve sustainability by decreasing the reliance on animals to obtain squalene in pharmaceutical preparations. Here we show that nanoemulsions comprised of Pinaceae-derived polyprenol oils demonstrated comparable physical stability and biological activity in various preclinical models compared to emulsions made with shark-derived squalene, particularly when formulated with the TLR4 ligand glucopyranosyl lipid adjuvant (GLA). Taken together, these results indicate that polyprenol-based nanoemulsions could be a suitable alternative to squalene-based nanoemulsions for influenza vaccine adjuvant applications.
Supplementary Material
Figure 7. Protective efficacy and functional antibody responses in male ferrets immunized with squalene or polyprenol nanoemulsions.
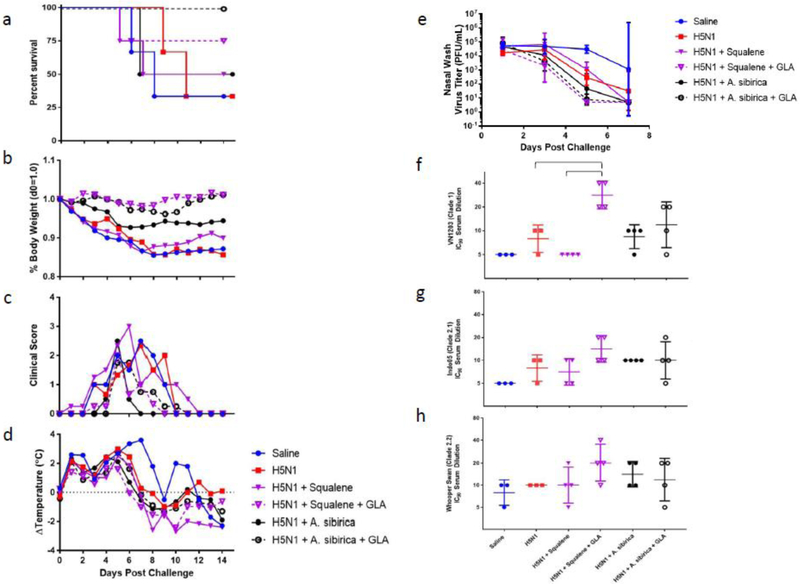
(a) Percent survival after a single immunization followed by intranasal challenge 21-days post immunization with A/Vietnam/1203/04. (b) Average normalized change in body weight following challenge. (c) Average clinical score following challenge. (d) Average change in temperature following challenge. (e) Geometric mean (+/− geometric s.d.) virus titer detected in nasal washes following challenge. (f-h) Geometric mean (+/− geometric s.d.) of virus neutralizing antibodies to homologous or heterologous strains measured 21 days after immunization using a retrovirus pseudotype neutralization assay.
Acknowledgments
The authors thank Sandra Sivananthan, Tony Phan, Tiep Pham, Adrian Simpson, Adeline Chen and Aaron Kahn for excellent technical assistance and Dr Vladimir Karpitskiy (PhD, Chemistry), Director of Research and Development for Solagift (subsidiary of Prenolica Ltd), for the preparation of the polyprenols. This work was supported by Contract #HHSO100201000039C from the Biomedical Advanced Research and Development Authority, Assistant Secretary for Preparedness and Response, Department of Human and Health Services and by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Human and Health Services under grant R01AI135673. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of ASPR/BARDA or NIH/NIAID.
Abbreviations:
- DMPC
dimyristoyl phosphatidylcholine
- GLA
glucopyranosyl lipid adjuvant
- HAI
hemagglutination inhibition
- MN
microneutralization
- TLR
toll-like receptor
Footnotes
Conflict of interest
The following declarations could be considered as potential conflicts of interest to this work: CBF, NVH, BG, SL, JAG, and DC are employees of IDRI, which has product assets based on TLR4 ligands and oil-in-water emulsions. VS is the principal founder and Executive Chairman of Prenolica, which has product assets based on polyprenols.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlington K, El harfi I, Li J, Carmichael K, Guderian JA, Fox CB, Irvine DJ, 2016. Molecular design of squalene/squalane countertypes via the controlled oligomerization of isoprene and evaluation of vaccine adjuvant applications. Biomacromol. 17, 165–172. [DOI] [PubMed] [Google Scholar]
- Belser JA, Katz JM, Tumpey TM, 2011. The ferret as a model organism to study influenza A virus infection. Dis Models Meehan 4, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito LA, Chan M, Baudner B, Gallorini S, Santos G, O'Hagan DT, Singh M, 2011. An alternative renewable source of squalene for use in emulsion adjuvants. Vaccine 29, 6262–6268. [DOI] [PubMed] [Google Scholar]
- Carter D, Fox CB, Day TA, Guderian JA, Liang H, Rolf T, Vergara I, Sagawa ZK, Ireton G, Orr MT, Desbien A, Duthie MS, Coler RN, Reed SG, 2016. A structure-function approach to optimizing TLR4 ligands for human vaccines. Clin Transl Immunol 5, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedotova I, Soultanov V, Nikitina T, Roschin V, Ordayn N, 2012. Ropren® is a polyprenol preparation from coniferous plants that ameliorates cognitive deficiency in a rat model of beta-amyloid peptide-(25–35)-induced amnesia. Phytomedicine 19, 451–456. [DOI] [PubMed] [Google Scholar]
- Fedotova I, Soultanov V, Nikitina T, Roschin V, Ordyan N, Hritcu L, 2016. Cognitive-enhancing activities of the polyprenol preparation Ropren® in gonadectomized β-amyloid (25–35) rat model of Alzheimer's disease. Physiol Behav 157, 55–62. [DOI] [PubMed] [Google Scholar]
- Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick T, 2008. Monitoring the effects of component structure and source and formulation stability and adjuvant activity of oil-in-water emulsions. Coll Surf B: Biointerfaces 65, 98–105. [DOI] [PubMed] [Google Scholar]
- Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS, 2011. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine 29, 9563–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CB, Barnes V,L, Evers T, Chesko JD, Vedvick TS, Coler RN, Reed SG, Baldwin SL, 2013. Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy. Influenza Other Respi Viruses 7, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CB, Haensler J, 2013. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 12, 747–758. [DOI] [PubMed] [Google Scholar]
- Misquith A, Fung M, Dowling QM, Guderian JA, Vedvick TS, Fox CB, 2014. In vitro evaluation of TLR4 agonist activity: formulation effects. Coll Surf B: Biointerfaces 113, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narovlyansky AN, Pronin AV, Sanin AV, Veselovsky VV, Danilov LL, Sedov AM, Ershov FI, 2018. Isoprenoids: Polyprenols and polyprenyl phosphates as physiologically important metabolic regulators. Nova Science Publishers, Hauppauge, NY. [Google Scholar]
- O'Hagan DT, Ott GS, De Gregorio E, Seubert A, 2012. The mechanism of action of MF59- an innately attractive adjuvant formulation. Vaccine 30, 4341–4348. [DOI] [PubMed] [Google Scholar]
- O'Hagan DT, Ott GS, Van Nest G, Rappuoli R, Giudice GD, 2013. The history of MF59 adjuvant: a phoenix that arose from the ashes. Exp Rev Vaccines 12, 13–30. [DOI] [PubMed] [Google Scholar]
- Oh DY, Hurt AC, 2016. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front Microbiol 7, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN, 2013. Adjuvant formulation structure and composition is critical for the development of an effective vaccine against tuberculosis. J Control Rel 172, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard NH, Frøsig TM, Weiner S, Rasmussen M, Ilsøe, Sørensen MR, Andersen MH, Buus S, Jungersen G, 2015. Establishing the pig as a large animal model for vaccine development against human cancer. Front Genet 6, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa O, Babeanu NE, Popa I, Nita S, Dinu-Parvu CE, 2015. Methods for obtaining and determination of squalene from natural sources. BioMed Res Int 2015, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschin VI, Soultanov VS, 2003. Method for processing vegetable raw materials, WO2004108848A1, RU2238291C1, US20060198910A1.
- Soultanov V, Fedotova I, Nikitina T, Roschin V, Ordyan N, Hritcu L, 2017. Antidepressant-like effect of Ropren® in β-amyloid-(25–35) rat model of Alzheimer's disease with altered levels of androgens. Mol Neurobiol 54, 2611–2621. [DOI] [PubMed] [Google Scholar]
- Soultanov VS, 2016. New hepatic and neurological clinical implications of long-chain plant polyprenols acting on the mammalian isoprenoid pathway. Eksp Klin Gastroenterol 135, 104–113. [PubMed] [Google Scholar]
- VanHoeven N, Fox CB, Granger B, Evers T, Joshi SW, Nana GI, Evans SC, Lin S, Liang EL, Liang L, Nakajima R, Feigner PL, Bowen RA, Marlenee N, Hartwig A, Baldwin SL, Coler RN, Tomai M, Elvecrog I, Reed SG, Carter D, 2017. A formulated TLR7/8 agonist is a flexible, highly potent and effective adjuvant for pandemic influenza vaccines. Sci Rep 7, 46426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherbee BM, Nichols PD, 2000. Lipid composition of the liver oil of deep-sea sharks from the Chatham Rise, New Zealand. Comp Biochem Physiol B: Biochem Mol Biol 125, 511–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.