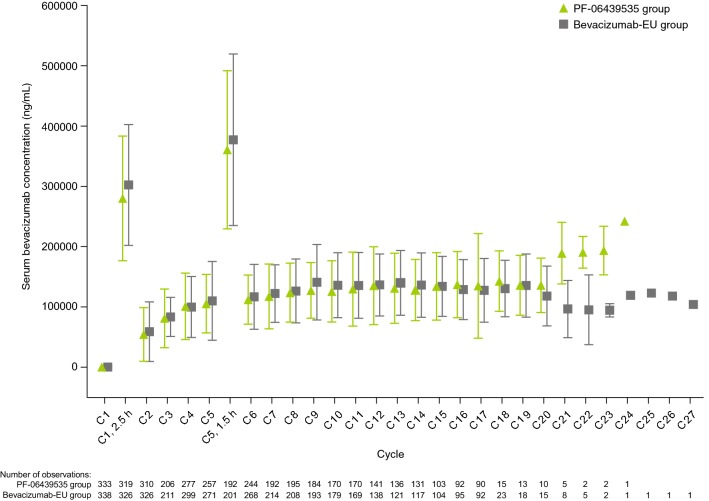
Fig. 4.
Mean serum concentrations of PF-06439535 and bevacizumab-EU in the PK population. Final data after study completion on 22 December 2017. Triangle or square and bar represent the mean with standard deviation. Concentrations are pre-dose (0-h time point) unless otherwise noted. Summary statistics calculated by setting concentration values below the LLOQ (< 250 ng/mL) to 0. Unplanned readings excluded. Samples with a time deviation of > 20% or any positive time deviation from the 0-h planned time point excluded. End of treatment and early withdrawal samples excluded. Standard deviation not shown for the 0-h time point at cycle 1, day 1 because the bars would be shorter than the height of the triangle and square symbols. There were 20 patients in the PF-06439535 group and 17 patients in the bevacizumab-EU group with measurable pre-dose concentrations above the LLOQ on cycle 1, day 1. Patients with measurable pre-dose concentrations > 5% of apparent Cmax (serum concentration at 2.5-h time point) on cycle 1, day 1 were excluded from the summary analysis. After this pre-specified exclusion, six patients in the PF-06439535 group and seven patients in the bevacizumab-EU group with measurable pre-dose concentrations on cycle 1, day 1 were included in the summary. Bevacizumab-EU reference bevacizumab sourced from the European Union, C cycle, Cmax maximum concentration, h hour(s), LLOQ lower limit of quantification, PK pharmacokinetics