Figure 1.
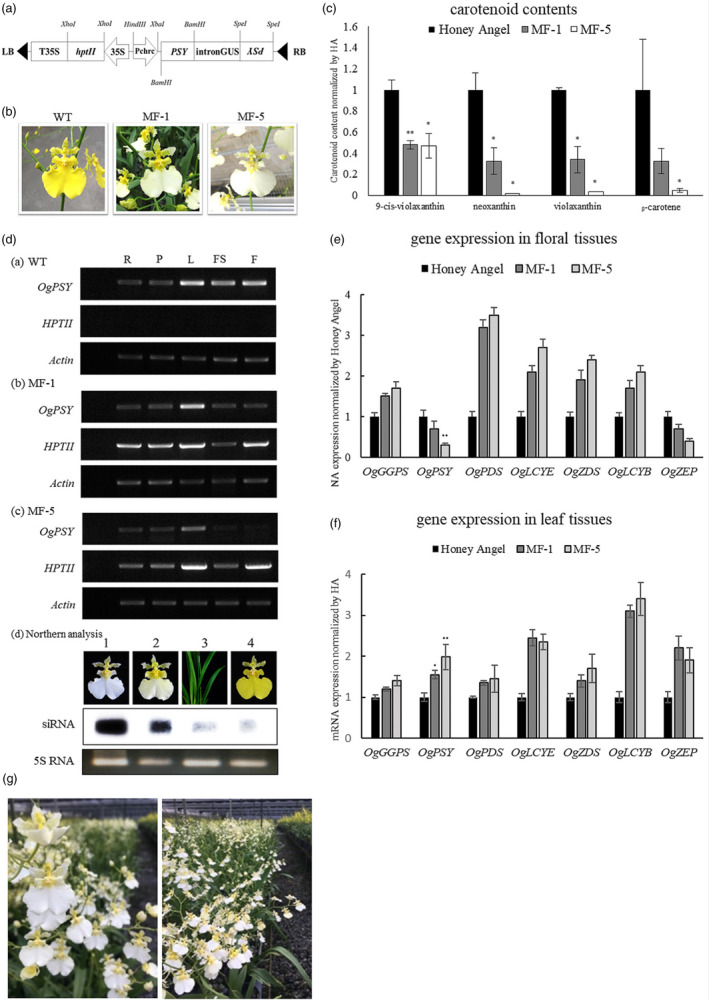
RNAi construct transformation, generation of white transgenic Oncidium varieties, and characterization of carotenoid compounds and relevant gene expression. (a) Schematic representation of hpRNA silencing vectors. Two 150‐bp PSY ORF fragments were inversely fused to both ends of the spacer DNA fragment, GUS intron (750 bp), and driven by Pchrc promoter. (b) Photographs of the transgenic Oncidium orchids harbouring PSY‐RNAi constructs. From left to right: WT orchid (yellow), transgenic orchid MF‐1 (yellowish white); and transgenic orchid MF‐5 (white). (c) Carotenoid levels in the floral tissues of the three Oncidium orchids. (*significantly different compared to control at P < 0.05, **significantly different compared to control at P < 0.01, by t tests, n = 2) (d) Analysis of OgPSY RNA expression in transgenic orchids. Semi‐quantitative real‐time PCR analysis of OgPSY expression in WT orchid; transgenic orchid MF‐1; and transgenic orchid MF‐5. R, root; P, pseudobulb; L, leaf; FS, flower stem; F, floral tissues; HPTII, hygromycin phosphotransferase II gene. Northern analysis of siRNA for the floral tissues of MF‐5 (lane 1), MF‐1 (lane 2), leaves of MF‐5 (lane 3) and the WT orchid (lane 4). The α‐32P‐labelled 18‐bp RNA probe, 5′‐GUUAGAUACACACCACGU‐3′, was used to hybridize. (e) and (f) Real‐time quantitative PCR analysis of the expression patterns of genes involved in carotenoid biosynthesis in floral tissues and in leaf tissues. (*significantly different compared to control at P < 0.05, **significantly different compared to control at P < 0.01, by t tests, n = 3) (g) The white transgenic orchid plants (MF‐5) grown in a greenhouse. They displayed the stable traits.
