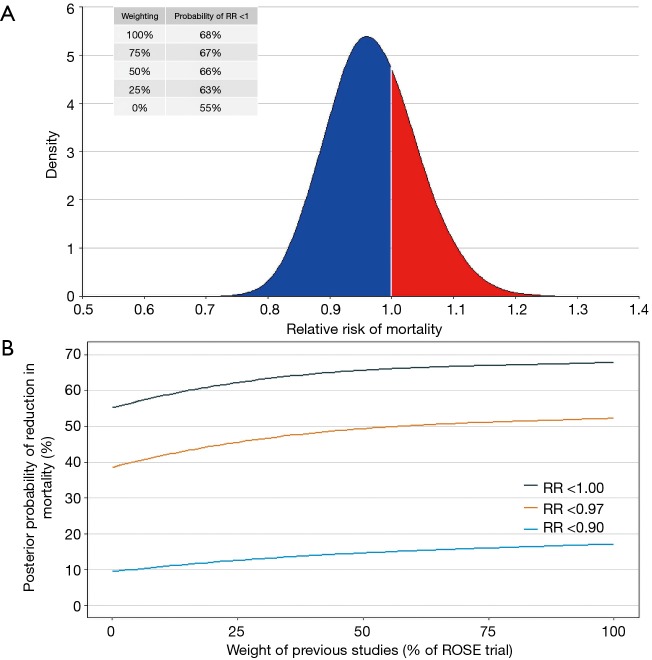
Figure 1.
Results of the Bayesian hierarchical meta-analysis. (A) Posterior probability distribution of the pooled results of the meta-analysis (full posterior density from the Markov chain Monte Carlo approach, the red area is where risk ratio >1 and NMBA increase mortality); (B) resulting estimated posterior probability that the risk ratio for mortality exceeds that threshold value according to different down-weightings of previous studies (if these weights are equal to 1, a meta-analysis of all studies are carried out, on the other hand, with the decrease in these weights, the importance of previous studies decrease and when these weights are equal to 0, then we learn nothing about the risk ratio for NMBA from previous studies, and with a minimally informative prior the results represent the analysis of the ROSE trial alone). ROSE trial, Reevaluation of Systemic Early Neuromuscular Blockade trial; NMBA, neuromuscular blocking agents.