Figure 2.
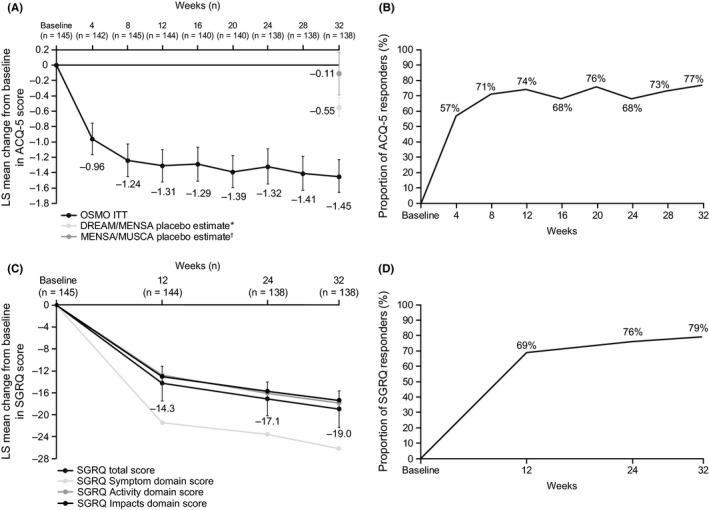
Analysis of (A) change from baseline in ACQ‐5 score, (B) the proportion of ACQ‐5 responders, (C) change from baseline in SGRQ total score, and (D) the proportion of SGRQ responders over the 32‐wk study period (ITT population). Vertical bars show 95% confidence intervals. *Placebo estimate of −0.55 (SE: 0.05), which was estimated from a meta‐analysis of studies MEA112997 (DREAM) and MEA115588 (MENSA) using all placebo patients; †Placebo estimate of −0.11 (SE: 0.14), which was estimated from a meta‐analysis of studies MEA115588 (MENSA) and 200862 (MUSCA) using placebo patients who previously used omalizumab. ACQ‐5 responders were defined as patients with ≥0.5‐point reduction from baseline; SGRQ responders were defined as patients with ≥4‐point reduction from baseline; Analyses were performed using mixed model repeated measures with covariates of region, baseline maintenance OCS therapy (OCS, no OCS), exacerbations in the prior year, and visit. ACQ‐5, Asthma Control Questionnaire; ITT, intent‐to‐treat; LS, least squares; OCS, oral corticosteroid; SE, standard error; SGRQ, St George's Respiratory Questionnaire
