Table 2.
Optimization of the 18F‐difluoromethylation reaction.
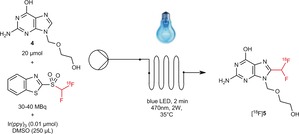
|
Entry |
Deviation from standard reaction conditions |
RCY [18F]5 [%] (of the crude reaction mixture) |
|---|---|---|
|
1 |
benzophenone[a] |
47±5 |
|
2 |
Ru(bpy)3 (0.01 μmol) |
0 |
|
3 |
– |
70±7 |
|
4 |
After HPLC purification of [18F]5 |
42±4[b] |
|
5 |
55 °C |
51±10 |
|
6 |
30 s |
60±8 |
|
7 |
Ir(ppy)3 (0.001 μmol) |
42±2 |
|
8 |
DMF |
44±1 |
|
9 |
DMSO/H2O (200/50 μL) |
45±10 |
|
10 |
no catalyst |
0 |
|
11 |
no light |
0 |
|
12 |
TEMPO |
0 |
[a] Benzophenone (10 μmol), 365 nm, [b] Radiochemical yield (n=4) of isolated product.
