Abstract
Older patients with acute myeloid leukemia (AML) have poor outcomes with standard induction chemotherapy. We retrospectively reviewed our institute’s experience with epigenetic (Epi) versus cytarabine- and anthracycline-based intensive chemotherapy (IC) as induction in newly diagnosed AML patients aged 60 years and older. One hundred sixty-seven patients (n = 84, IC; n = 83, Epi) were assessed; 69 patients received decitabine and 14 azacitidine. Baseline characteristics between the IC and Epi patient cohorts were not statistically different except for age, initial white blood cell count, and comorbidity index. Overall response rate (ORR, 50% vs. 28%, respectively, P< 0.01) and complete response rate (CRR, 43% vs. 20%, respectively, P< 0.01) were superior following IC vs. Epi. Although univariate analysis demonstrated longer overall survival after IC (10.7 vs. 9.1 months, P = 0.012), multivariate analysis showed no independent impact of induction treatment. Treatment-related mortality was not statistically different in the two groups. Outcomes of patients with secondary, poor cytogenetic risk, FLT-3 mutated AML, or relapsed/refractory disease after IC or Epi were not significantly different. Outcomes of patients receiving IC versus a 10-day decitabine regimen (n = 63) also were not significantly different. Our results suggest that IC and Epi therapy are clinically equivalent approaches for upfront treatment of older patients with AML and that other factors (feasibility, toxicity, cost, etc.) should drive treatment decisions. Prospective randomized trials to determine the optimal induction approach for specific patient subsets are needed.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy occurring primarily in older adults, with a median age at onset of 70 years [1,2]. Development of AML in patients aged 60 years and older is termed elderly AML and is recognized as a distinct clinical entity from AML in younger adults or children. Prognosis in elderly AML is worse compared to younger patients due to specific biological characteristics associated with chemoresistance [3]. These include a higher incidence of unfavorable cytogenetics (monosomy 7, del 5q-/monosomy 5, and complex abnormalities), frequent involvement of a more immature leukemic precursor clone, high rates of antecedent hematopoietic disorders, and expression of multidrug-resistant proteins (such as P-glycoprotein) [3–5]. Older AML patients are also more likely to have poor performance status and increased comorbidities, which contribute to higher treatment-related mortality and morbidity [6–8]. Taken together, these factors make the treatment of elderly AML very challenging.
Consequently, the appropriate selection of treatment for elderly AML is controversial. Conventional therapy for elderly AML patients (short of clinical trials) consists of intensive chemotherapy (IC) with cytarabine- and anthracycline-based regimens, non-intensive chemotherapy with low-dose cytarabine, or supportive care with hydroxyurea and transfusion support. The appropriate choice among these strategies depends on prognostic scoring and clinical assessment of performance status in older AML patients [9]. Although IC remains the standard of care for all AML patients, only 30–60% of older patients receive it. Five-year survival rates are as low as 15% in such individuals [6,7,10]. These statistics highlight a major unmet need for new treatment options in the management of newly diagnosed elderly AML.
Epigenetic agents that inhibit promoter DNA methylation [i.e., hypomethylating agents (HMA)] have shown potential in the treatment of newly diagnosed elderly AML [11,12]. HMA inhibit epigenetic silencing of cell-cycle regulatory elements and tumor suppressor genes, thus suppressing the reversible epigenetic changes that play a major role in pathogenesis of leukemogenesis [13–16]. Two agents, decitabine (Dec) and azacitidine (Aza), have been approved for the treatment of patients with myelodysplastic syndrome (MDS) and have been well tolerated in elderly individuals, leading to multiple clinical trials evaluating their use in older AML patients [17–32]. The earliest clinical data of HMAs in AML patients were noncomparative trials mostly involving individuals unfit for IC [17–20,25]. Next, subgroup analyses of large prospective phase III trials of Aza or Dec demonstrated superior outcomes for HMA over best supportive care in AML patients with marrow blast counts of 20–30% [21,30,33]. HMA have subsequently been evaluated in multiple trials for elderly AML patients with more aggressive disease. To date, the most convincing data are from a randomized phase III trial of Dec vs. either supportive care or low-dose cytarabine (LDAC) in elderly AML patients with intermediate or poor cytogenetic risk disease. Primary analysis of this trial showed a clinically superior but nonsignificant overall survival (OS) advantage to Dec over LDAC therapy (7.7 vs. 5.0 months; HR: 0.85, 95% CI: 0.69–1.04; P = 0.108). An unplanned secondary analysis with longer follow-up has since demonstrated a statistically significant difference in OS for Dec-treated patients [34].
Here, we assessed the outcomes of epigenetic (Dec and Aza) and standard infusional cytarabine- and anthracycline-based “7+3” chemotherapy as induction therapy for newly diagnosed, elderly AML patients. This retrospective study was supported by a convenience sample from a single institution. Patients at least 60 years old on the date of AML diagnosis (either de novo or secondary) were included, regardless of initial blast count, fitness for chemotherapy, performance status, or cytogenetic risk category.
Materials and Methods
Patients and treatment
We retrospectively reviewed all consecutive available electronic medical charts of 167 adults aged 60 years and older with newly diagnosed AML who received induction therapy at Roswell Park Cancer Institute (RPCI) from March 2008 to February 2013. The study was approved by our institutional review board (IRB) and was carried out according to the principles of the Declaration of Helsinki. All patients who received either intensive chemotherapy (IC) or epigenetic (Epi) therapy as induction treatment were eligible for this study. Treatment comprised approved treatment regimens given at the discretion of the treating physician or administered as a part of an available clinical trial. Choice of induction therapy was based on clinical trial eligibility or, if no trial was available, per discretion of the primary oncologist. All patients who received induction therapy provided written informed consent for trial participation or to receive standard chemotherapy. For study inclusion, patients were required to have newly diagnosed AML as defined by WHO criteria [bone marrow (BM) or peripheral blood (PB) blasts ≥ 20%]. Both de novo and secondary AML patients were included. Diagnostic AML samples were tested for the presence of NPM-1 and FLT-3 mutations. Patients in the IC group received infusional cytarabine (100 mg/m2/d) intravenously for 7 days and daunorubicin (60 mg/m2/d) or an equivalent anthracycline intravenously for 3 days. Patients in the epigenetic (Epi) therapy group received Dec intravenously at 20 mg/m2/d over 1 hr for 5 or 10 consecutive days or subcutaneous or intravenous Aza at 75 mg/m2/d for 7 days at planned 4-week intervals (see Supporting Information Table I for additional details of treatment regimens).
Assessment of efficacy and safety
All patients who initiated at least one cycle of Epi or IC were included in the efficacy and safety analysis. The efficacy end points were OS, leukemia-free survival (LFS), complete response rate (CRR), overall response rate (ORR), proportion of patients receiving hematopoietic stem cell transplantation (HSCT), and relapse rate (RR). The ORR included rates for complete marrow response with complete hematological response (CR), complete response with incomplete leukocyte or platelet recovery (CRi), and partial response (PR). Treatment response was assessed using the 2003 revised International Working Group response criteria [35]. The safety end points studied were 30- and 60-day mortality after initiation of induction therapy from any cause.
Statistical analysis
Baseline imbalances between the treatment groups were assessed descriptively and with a multivariable logistic regression model. Logistic regression was also used to assess the effect of treatment on overall response (OR). The treatment effect was described using the odds ratio and 95% CI estimates. This association was also explored in a competing risks framework, with OR as the event of interest and death as the competitive state [36]. The failure time outcome was defined as the number of months between AML diagnosis and documentation of OR or death, whichever came first. Patients with neither event were censored at the date of last follow-up. Following the example of Latouche et al. [37], both cause-specific hazards and cumulative incidence function subdistribution hazards results were estimated with proportional hazards models [38]. The cause-specific and subdistribution hazards are sometimes referred to as the “rate” and the “risk,” respectively.
The effect of treatment on the chance of HSCT was modeled using competing risks methods, with death as the competing risk. The failure time outcome was defined as the number of months between AML diagnosis and HSCT or death, whichever came first. Patients with neither event were censored at the date of last follow-up. Proportional hazards estimates of the cause-specific and subdistribution hazards were used to describe the association between treatment type and outcomes.
The effect treatment on OS was described using proportional hazards modeling with and without HSCT as a time-dependent covariate. OS was defined as the number of months between initiation of Epi or IC and death from any cause. Among patients with a documented CR, LFS was defined as the time from CR to relapse or death, whichever was earlier. Patients without documentation of the given event were censored at the date of last follow-up. Time-to-event outcomes were also described with Kaplan–Meier curves, with treatment differences assessed by the log rank test. Median follow-up time was estimated by the reverse Kaplan–Meier method.
Final multivariable models were obtained by reducing a full model containing several patient and disease characteristics as possible confounders of the treatment effect estimate. These factors were eliminated from the model using backward selection with a P value retention threshold of 0.30. The induction therapy indicator was retained regardless of P value. Descriptive analyses of treatment differences across categorical factors used Fisher’s exact test. Between-treatment differences of continuous variables were compared by the Wilcoxon rank sum test. The results of this single-institution, convenience sample study were considered hypothesis generating only. P values <0.05 were considered statistically significant, with no multiple testing adjustment to control the overall Type I error rate.
All data analyses were generated using SAS/STAT software, version 9.4 (Copyright 2012, SAS Institute, Inc. SAS is a registered trademark of SAS Institute, Inc., Cary, NC). Details of statistical methods and results are described in Supporting Information Statistical Methods.
Results
Baseline characteristics
A total of 167 patients treated at RPCI between March 2008 and February 2013 met the criteria for inclusion. Median age of patients was 70 years (range, 60–92 years) with baseline marrow blasts of 52%. Eleven percent of all patients (n = 18) showed AML characterized by FLT-3 mutations and 15% (n = 23) NPM-1 mutated AML. Of the 18 patients with AML characterized by FLT-3 mutations, 13 showed FLT-3 ITD and five FLT-3 D835 mutations. None of the patients had both the mutations. Almost half (82/167, 49%) had secondary AML, and 47% had documented poor-risk cytogenetics. Half of the patients (84, 50%) received IC, and half (83 patients, 50%) received Epi therapy. Among the Epi group, 69 patients (83%) received Dec and 14 patients (17%) received Aza. The majority of patients treated with Dec (63/69, 91%) received a 10-day decitabine regimen. Six patients received a 5-day decitabine regimen due to enrollment on specific protocols (n = 2) or physician decision (n = 4).
Imbalances between IC and Epi groups included baseline age, white cell count (WBC) at treatment initiation, and comorbidity index. Patients receiving Epi therapy were approximately 8 years older than those receiving IC (mean, 75.5 vs. 67.5 years, P < 0.001). Patients in the IC treatment group also had higher WBC than the Epi group (12K vs. 5K, P = 0.03) with a trend to higher PB blasts (median 21% vs. 9%, P = 0.06). More patients in the IC group proceeded onto subsequent HSCT than Epi patients (P < 0.001). We found no differences in the proportion of patients with secondary AML (36% vs. 46%, P = 0.12), poor-risk cytogenetics (29% vs. 40%; P = 0.24), Eastern Cooperative Oncology Group (ECOG) performance status 0–1 (85% vs. 82%, P = 0.68), FLT-3 mutation (15% vs. 7%, P = 0.21), NPM-1 mutation (19% vs. 10%, P = 0.17), or BM blasts at diagnosis (53% vs. 45%, P = 0.18) (Table II). Detailed analysis of comorbidities as assessed by the Charlton comorbidity index (CI) demonstrated that patients receiving IC had significantly fewer comorbidities as reflected by lower CI scores (P < 0.001) (Supporting Information).
Table II.
Multivariate Analysis of Prognostic Factors for Overall Survival in Older AML Patients
| Groups | Hazard ratio | 95% CI | P | |
|---|---|---|---|---|
| Induction therapy | IC (vs. Epi) | 0.775 | 0.497, 1.207 | 0.26 |
| Age | - | 1.053 | 1.025, 1.083 | 0.0002 |
| Charlson score | 1.028 | 0.920, 1.149 | 0.63 | |
| Cytogenetic risk category | Good/intermediate (vs. poor risk) | 0.906 | 0.582, 1.411 | 0.66 |
| Sex | M (vs. F) | 1.101 | 0.719, 1.686 | 0.66 |
| ECOG PS | 0–1 (vs. 2–4) | 0.454 | 0.275, 0.751 | 0.002 |
| WBC@diagnosis | - | 1.005 | 0.997, 1.012 | 0.21 |
| Hemoglobin@diagnosis | - | 1.022 | 0.903, 1.158 | 0.73 |
| Platelets@diagnosis | - | 0.999 | 0.996, 1.001 | 0.22 |
| BM blasts@diagnosis | - | 0.991 | 0.980, 1.003 | 0.13 |
| PB blasts@diagnosis | - | 1.011 | 1.001, 1.022 | 0.036 |
| FLT3-mutant | No (vs. yes) | 1.625 | 0.781, 3.385 | 0.19 |
| NPM1-mutant | No (vs. yes) | 0.882 | 0.457, 1.701 | 0.71 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; IC, intensive chemotherapy; Epi, epigenetic therapy; CI, confidence interval.
Comparison of outcomes of IC versus Epi induction therapy
Objective response
In the IC group, 42 (50%) patients had an OR to treatment with 36 (43%) CR and six (7%) CRi reported. In the Epi group, 23 (28%) patients showed an OR with 16 (20%) CR and seven (8%) CRi. The difference in ORR and CR between the two treatment groups was statistically significant (ORR, P = 0.001; CR; P = 0.004) (Supporting Information Table II). Accounting for the relative timing of the possible outcomes, we compared the chance of response and death in the patients in IC (ref: Epi) using competing risks modeling. As confirmed in the multivariable analysis [cause-specific HR for ORR = 2.96 (1.55 to 5.65), subdistribution HR = 2.23 (1.23 to 4.02)], the IC patients had higher rates of response. The effects of treatment on the time to death in the presence of ORR were not statistically significant (Supporting Information Statistical Methods).
Overall survival
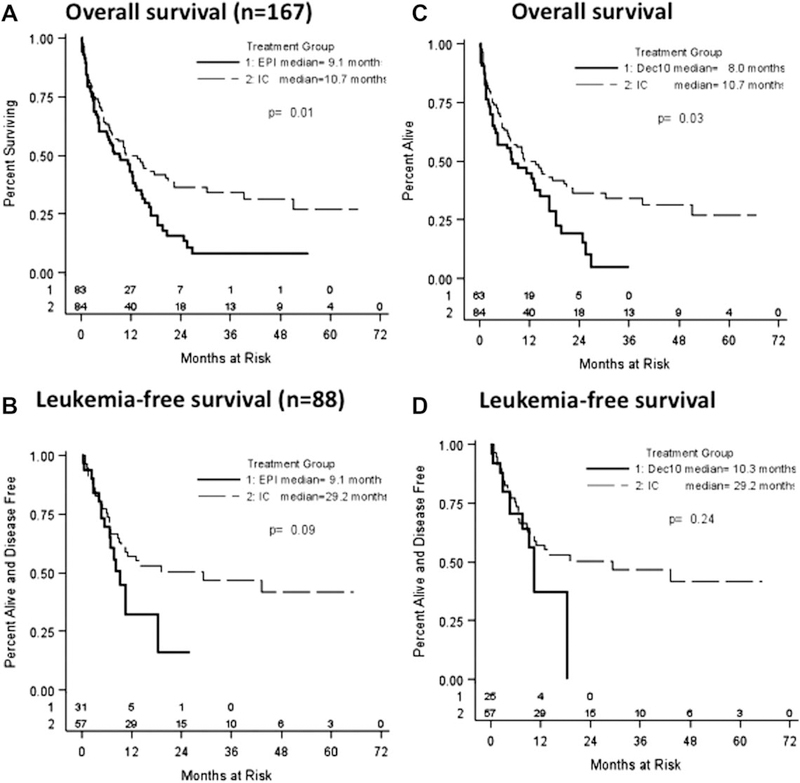
In the univariate analysis, the median OS (95% confidence interval) following IC was significantly higher than after Epi therapy (10.7 vs. 9.1 months) (P = 0.012) (Fig. 1A). There was also a trend to prolonged LFS with IC therapy (29.2 vs.9.2 months, P = 0.09) (Fig. 1B). Median follow-up of all patients was 24.9 months.
Figure 1.
Outcomes of older AML patients treated with different induction strategies. (A) Overall survival of older AML patients following induction with intensive chemotherapy (IC) or epigenetic (Epi) therapy. Median survival of the IC group was 10.7 months as compared with 9.1 months in the Epi-treated patients (P = 0.012). (B) Leukemia-free survival of patients following IC or epigenetic therapy. Median survival of IC group was 29.2 months as compared with 9.2 months (P = 0.090). (C) Overall survival of older AML patients following induction with IC or 10 days of decitabine (Dec10). Median survival of the IC group was 10.7 months as compared with 8.0 months in the Dec10-treated patients (P = 0.026). (D) Leukemia-free survival of patients following induction with IC or Dec10. Median survival time of IC group was 29.2 months as compared with 10.3 months following Dec10 treatment (P = 0.239). Epi (solid line), epigenetic therapy; IC (dotted line), intensive chemotherapy; median, median overall survival.
Receipt of HSCT
As described earlier, the risk of HSCT or death was modeled using competing risks methods.
We found that IC patients (ref: Epi) were more likely to have an HSCT before dying in the univariate analysis. This association was maintained in the multivariable models [HSCT cause-specific HR = 2.51 (1.03 to 6.14), subdistribution HR = 2.02 (0.80 to 5.07)]. These HR estimates are quite strong but not statistically significant (Supporting Information Statistical Methods).
Impact of HSCT on outcomes of induction therapy
Thirty-three (20%) of the 167 patients underwent HSCT subsequent to induction therapy, with the majority disproportionately having received upfront IC, not Epi, induction (Table II). Baseline characteristics showed persistent imbalances in age and initial WBC count between the two induction groups (data not shown). As HSCT was more likely to be performed in younger AML patients in CR and may result in improved OS, we repeated our analysis with and without adjustment for HSCT status. Despite a significantly higher CR rate following IC (52% IC vs. 25% Epi, P = 0.002), the univariate analysis demonstrated no statistically significant difference in the OS of these older nontransplanted AML patients based on induction regimen (5.4 IC vs. 7.5 months Epi; P = 0.415) (data not shown). One-year survival was also similar (34.1% IC vs. 36.2% for Epi-treated patients). Survival of patients with censoring at the time of HSCT was also performed (Supporting Information Fig. 1).
A major issue regarding successful transplantation of older AML patients is remission status at the time of HSCT. Despite lower ORRs following Epi therapy, over 80% of patients in each induction group had no morphological evidence of AML at the time of transplant, in some cases, due to administration of additional interim chemotherapy. Twenty-two of 26 IC patients (85%) and six of 7 (86%) Epi patients were in CR/CRi. All of the four Dec-treated patients (three CR and one CRi) and two of the three Aza-treated patients were in CR/CRi. The remaining patients were transplanted in PR with 5.6 to 23% residual marrow blasts.
Multivariable analysis for prognostic factors
The effect of induction therapy (IC vs. Epi) on OS was assessed using a multivariable proportional hazards model, adjusting for age, Charlson comorbidity score, PB blasts at diagnosis, WBC at diagnosis, BM blasts, FLT-3 mutation, and platelet count at diagnosis. HSCT was excluded from the covariate set or included as a time-dependent covariate (Supporting Information Statistical Methods). The estimated prognostic effect of IC (ref: Epi) indicated a 24% reduction in the rate of death, but the effect was not statistically significant [HR = 0.76 (0.49 to 1.17); P = 0.21 without HSCT adjustment]. HSCT represented a 23% reduction in the risk of death after adjusting for other factors but was also not statistically significant [HR = 0.77 (0.50 to 1.20); P = 0.25). These data demonstrate that OS in older patients with AML was not significantly associated with either induction therapy choice or receipt of HSCT. Improved outcomes were associated with younger age of patients, better ECOG, lower comorbidity, and lower PB blasts at diagnosis (Table II). Results of this multivariable model were further confirmed using propensity score adjustment methods (Supporting Information Statistical Methods).
Toxicity
We studied treatment-related toxicity as reflected by 30- and 60-day mortality following initiation of induction therapy. Differences in mortality based on IC vs. Epi treatment regimens at either time point were not statistically significant. Nine patients (11%) in the IC group and nine patients (11%) in the Epi group died within 30 days of starting induction treatment (P = 1.0). A total of 15 patients in the IC group (18%) and 17 patients in the Epi group (21%, P = 0.69) died within 60 days of induction initiation. Causes of death were similar (Supporting Information Tables II and III).
Subgroup analysis
Several subgroup analyses were performed to assess the impact of other potential variables on the outcome of induction treatment.
Comparison of outcomes of Aza versus Dec
Among the 83 patients in the Epi induction group, 69 (83%) received Dec and 14 (17%) received Aza. Although baseline characteristics were not statistically significant between the two groups, Aza-treated patients tended to have lower BM blasts and a higher rate of adverse cytogenetics than either the Dec or IC induction cohorts (Supporting Information Table IV). However, differences in OS (Dec 9.4 vs. Aza 6.8 months, P = 0.523), LFS (9.2 vs. 6.1 months, P = 0.802), and response rates (27% vs. 29%, P = 1.00) between the Aza- and Dec-treated patients were not statistically significant (Supporting Information Table V and Fig. 2).
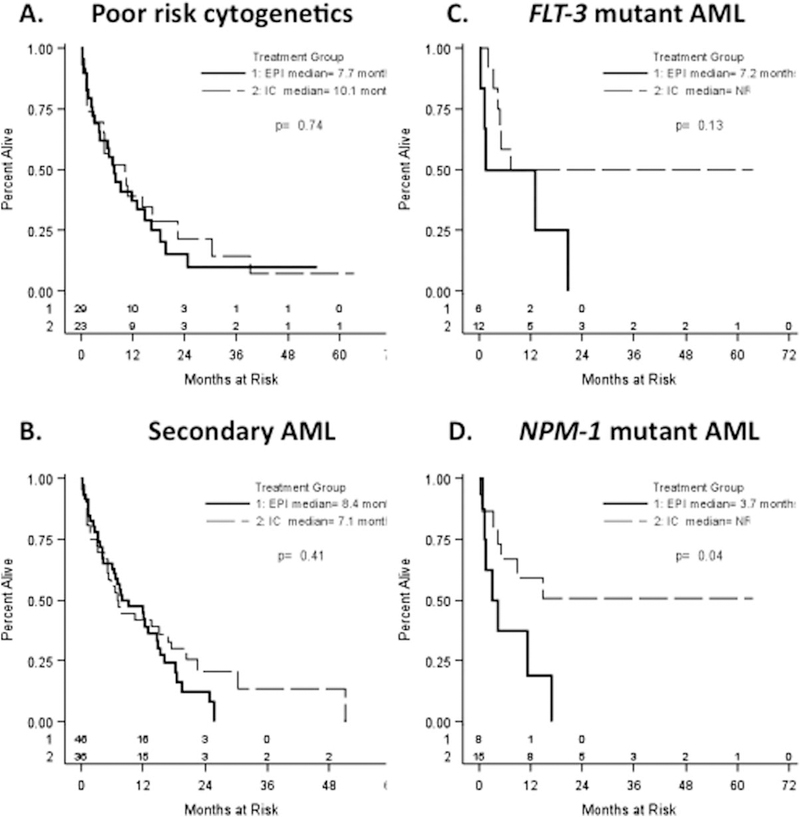
Figure 2.
Impact of induction therapy on survival of subgroups of older AML patients. Overall survival of patients defined by (A) poor cytogenetics; (B) secondary AML; (C) presence of FLT-3 mutations; and (D) presence of NPM-1 mutations are shown. Epi (solid line), epigenetic therapy; IC (dotted line), intensive chemotherapy; median, median overall survival; NR, not reached.
Comparison of outcomes of IC versus10-day Dec
The majority (63/69) of Dec-treated patients received induction with a 10-day decitabine regimen (Dec10). We, therefore, repeated our analyses to specifically compare the outcomes of AML patients receiving IC vs. Dec10 and omitting those patients receiving Aza or 5-day Dec therapy. The IC/Dec10 choice outcome was described by a logistic regression model specified to include meaningful clinical and demographic covariates. Details of the statistical methods are included in the Supporting Information Statistical Methods. Patients receiving IC tended to be younger (odds ratio per 1 year increase: 0.84, 95% CI: 0.79, 0.90; P < 0.01) with higher WBC (odds ratio per unit increase 1.03, 95% CI: 1.01, 1.05; P < 0.01) than those receiving Dec10. Other differences between the two groups, including performance status and PB blast percentages, were not statistically significant (data not shown).
Next, we examined response IC (ref: Dec10) patients, adjusting for induction therapy and baseline clinical factors. Patients receiving IC had significantly higher odds of response [adjusted odds ratio = 2.44; (1.07 to 5.57; P = 0.03] as reflected in a 50% ORR as compared with Dec10 therapy (ORR of 28%) (Supporting Information Table VI). Consistent with the higher response rate, IC patients also were more likely to receive an HSCT, but the difference was not statistically significant [odds ratio = 3.08 (0.85 to 11.13) P = 0.09]. Overall, patients receiving HSCT tended to be younger (P < 0.01) and to have better ECOG performance status (P = 0.045) than those not undergoing HSCT. After controlling for the other factors, response was not an independent predictor of HSCT (P = 0.95).
In the univariate analysis of survival outcomes, IC demonstrated significantly longer OS than Dec10 (10.7 vs. 8.0 months, P = 0.03) (Fig. 1C). However, differences in LFS between the two groups (IC 29.2 vs. Epi 10.3 months, P = 0.24) (Fig. 1D) or median OS when patients undergoing HSCT were excluded and were not statistically significant (data not shown).
The effect of induction therapy (IC ref: Dec10) on OS was also evaluated using a multivariable proportional hazards model. Similar to our prior analysis of IC vs. Epi, this model was adjusted for age, Carlson score, cytogenetic risk, BM blasts, and PB blasts. HSCT status was modeled as a time-dependent covariate. After adjusting for these factors, patients receiving IC therapy tended to have better OS than the Dec10 patients, but this difference was not statistically significant (HR: 0.76 95% CI: 0.48 to 1.20; P = 0.23). Receipt of an HSCT after the start of induction therapy provided some benefit, but again, the effect was not statistically significant (P = 0.33). Improved survival was identified in younger patients, those with an ECOG performance status of 0–1, and lower PB blasts with a trend for lower BM blasts (Supporting Information Table VI).
Impact of poor-risk cytogenetics on outcome stratified by choice of induction
Since cytogenetic findings are the major prognostic indicator of response to standard 7 + 3 chemotherapy, we studied the effect of IC vs. Epi in the subgroup (n = 54 of 167, 35%) of AML patients with poor-risk cytogenetics. Of these patients, 23 (29%) received IC, whereas 31 (40%) received Epi. Median OS was 10.1 months (2.9 to 16.7) following IC and 7.7 months (3.1 to 14.9) following Epi (P = 0.706) (Fig. 2A). ORRs were 43% after IC vs. 32% following Epi (P = 0.57) (Supporting Information Table VII). These differences were not statistically significant.
Impact of secondary AML on outcome of choice of induction
Among our patients, 82 (49%) had known secondary AML at diagnosis. Thirty-six (43%) patients received IC, whereas 46 (55%) received Epi therapy. Median OS was 7.1 months (4.4, 16.7) following IC and 8.5 months (4.3, 14.6) following Epi (P = 0.407) (Fig. 2B). Objective responses were 44% after IC vs. 30% after Epi (P = 0.25) (Supporting Information Table VII). These outcomes were not statistically significant.
Impact of molecular genetics on outcome stratified by choice of induction
In normal karyotype AML, the presence of a FLT-3 ITD mutation is independently associated with poor prognosis, while NPM-1 mutation without FLT-3 ITD mutation correlates with favorable prognosis. We studied the impact of these genetic mutations on the outcomes of our patients following specific induction treatment. The percentages of AML patients with FLT-3 or NPM-1 mutations were <20% in both the IC and Epi induction arms. Eighteen patients tested positive for FLT-3 mutation: 12 patients in the IC group and six patients in the Epi group (P = 0.21). The OS of FLT-3 mutant patients following each induction is shown in Fig. 2C and was not statistically significant (P = 0.13). Twenty-three patients tested positive for NPM-1 mutations. Of these, 15 patients received IC, and eight received Epi therapy (P = 0.17). Median OS in NPM-1 mutant patients following IC could not be calculated as the upper limit of OS was not reached. In contrast, the median OS following Epi therapy was 3.7 months, and this difference in survival was statistically significant (P = 0.038) (Fig. 2D). Higher response rates among NPM-1 mutant patients were also noted following IC vs. Epi therapy (80% vs. 25%) and were also statistically significant (P = 0.02) (Supporting Information Table VII).
Impact of induction therapy on subsequent salvage therapy outcome
Development of relapsed and/or refractory AML (RR-AML) following standard IC may predict for resistance as the same or similar cytotoxic agents used in the 7 + 3 induction are often employed in the salvage setting. We therefore analyzed outcomes of RR-AML patients to determine the potential effect of induction treatment type on subsequent response and survival rates. A total of 56 patients (43% of 167 patients) received salvage therapy for RR-AML. Thirty-two (57%) received IC and 24 (43%) received Epi therapy for primary induction. Differences in response rates (28% IC vs. 37% Epi; P = 0.57) (Supporting Information Table IV) and median OS (14.2 IC vs. 14.9 months, Epi; P = 0.747) (Supporting Information Fig. 3) based on the choice of induction therapy were not statistically significant among these RR-AML patients.
Discussion
AML is primarily a disease of the elderly with a median age of diagnosis of 67–70 years. Currently, there is no consensus on the optimal treatment for older patients with AML. Epidemiological and clinical studies have consistently demonstrated that AML patients up to 80 years old who are treated with intensive induction chemotherapy survive significantly longer than individuals receiving palliative care [39,40]. Despite this, many older individuals continue to be offered only low intensity or best supportive care due to concerns about treatment-related mortality and morbidity with intensive regimens. In recent years, the HMAs, decitabine or azacitidine, have emerged as an alternative induction strategy for older AML patients [41]. Prior studies have demonstrated that HMA therapy results in equivalent or potentially superior outcomes over low-dose cytarabine or best supportive care in older AML patients deemed unsuitable for intensive chemotherapy [34,42]. To date, however, the question of whether fit older individuals with AML should be offered epigenetic therapy in place of standard intensive chemotherapy remains unclear.
Here, we present our single-institute experience comparing the outcomes of epigenetic vs. standard 7 + 3 induction therapy in 167 patients aged 60 years and older treated for AML at our institute between 2008 and 2013. Our study population represented largely high-risk AML patients with a median age of 70 years, high baseline marrow blasts (52%), high percentages of secondary AML (49%), and poor-risk cytogenetics (47%). The overwhelming majority of AML patients in both induction groups had a performance status of 0–1 (85% vs. 82%, P = 0.68), suggesting they were appropriate candidates for definitive AML therapy. Of note, patients treated with IC vs. Epi induction therapy were significantly imbalanced for initial WBC, age, and comorbidities marrow blasts (52%), high percentages of secondary AML (49%), and poor-risk cytogenetics (47%). The overwhelming majority of AML patients in both induction groups had a performance status of 0–1 (85% vs. 82%, P50.68), suggesting they were appropriate candidates for definitive AML therapy. Of note, patients treated with IC vs. Epi induction therapy were significantly imbalanced for initial WBC, age, and comorbidities.
Overall, we found no independent impact of induction choice on clinical outcomes. In the univariate analysis, IC was associated with higher ORR (50% vs. 28%, P = 0.001), CRR (43% vs. 20%, P = 0.004), and median OS (10.7 vs. 9.1 months, P = 0.012) as compared with Epi therapy [7,21,43,44]. However, when we performed a Cox proportional hazards regression model controlling for the baseline differences in patient characteristics and accounting for HSCT as a time-dependent factor, we found no statistically significant difference in OS outcome based on induction choice (IC vs. Epi). In fact, the observed differences in OS in the two induction cohorts could be attributed to significant differences in age, performance status, and PB blasts.
Consistent with prior retrospective studies, the decision to administer intensive vs. epigenetic induction therapy in these older AML patients was driven largely by physician perceptions of patient fitness and disease biology [27,42,45]. In 1987, Neuss et al. identified six factors favoring definitive therapy over palliative care in AML patients: treatment on protocol, younger age, presence of dependent children, male sex, lack of antecedent hematologic disorder, and physician preference. They emphasized that, in most cases, the treating physician, rather than the patient, was the main therapeutic decision maker [46]. Since then, multiple models prognosticating the outcomes of older AML patients receiving IC have been developed. While there are some differences, the cornerstone of all of these models remain age, performance status, and cytogenetics [7,47]. Tools to more accurately judge the fitness of older AML patients independent of chronological age have been developed. These include indexes of other comorbidities and overall functionality on geriatric fitness assessment [48]. Disease aggressiveness also impacts on therapy choice. When Lowenberg et al. [39] randomized older AML patients to a “wait and see” approach instead of chemotherapy, they found that rapid disease progression leading to hyperleukocytosis and thrombocytopenia often necessitated initiation of cytoreductive therapy. Our study, as well as others, demonstrated that patients with high levels of surrogate markers reflective of increased cell turnover and tumor burden (specifically initial WBC, serum lactate dehydrogenase, and PB or BM blasts) preferentially receive IC as opposed to HMA or other low-intensity therapy [27,42].
Other features of AML disease, specifically preexisting MDS or therapy-related disease and prognostic gene mutations, have increasingly been used to determine choice of induction regimen. We therefore performed subgroup analyses addressing whether specific subsets of patients benefited from IC vs. Epi therapy. We found no statistically significant differences in the outcomes of older AML patients treated with either induction approach when stratified for poor-risk cytogenetics, secondary disease, or presence of FLT-3 mutations. The only exception was patients with AML characterized by NPM-1 mutations who exhibited improved survival following IC therapy, as previously reported [49,50]. Outcomes of patients with subsequent relapsed or primary refractory AML also were not statistically different based on initial induction approach. While intriguing, these results should be interpreted cautiously given the small sample sizes available for subgroup analysis and the fact that this was a single-center retrospective study. Further validation in larger prospective studies is needed.
Despite the preconception that HMA are less toxic than IC in older AML patients, we found that treatment-related mortality was not statistically different between the two induction groups, as reflected by the early death rates at 30 (11% IC vs. 11% Epi, P = 1.0) and 60 days (18% IC vs. 21% Epi, P = 0.69) after treatment initiation. Causes of death, mostly infectious complications, were also similar. Multiple prior studies examining the effects of decitabine in older AML patients reported 30-day mortality rates ranging from 2% to 32%, potentially reflecting the heterogeneous older patient population [19,51,52]. Our study did not measure other parameters such as number of required transfusions or inpatient days, both of which have been reported to be significantly lower following Epi vs. IC therapy [27]. These quality of life factors as well as the older age and lower WBC of the patients treated with Epi therapy may imply an advantage to HMA in select older patients as opposed to intensive therapy.
Our results contribute to a growing body of literature supporting HMA as a standard treatment approach for older patients with AML. Quintás-Cardama et al. performed a retrospective study of older AML patients treated with intensive vd. hypomethylating induction therapy at MD Anderson Cancer Center (MDACC). Patients receiving IC vs. Epi induction were significantly imbalanced for age, hemoglobin, and PB and BM blasts. As compared with our study, almost all patients in the MDACC study were enrolled on clinical trials, and almost half of the IC-treated patients received high-dose cytarabine (i.e., cytarabine 1.5 g/m2/d for 3 days) induction instead of a 7-day infusional cytarabine regimen. Although patients receiving either Aza or Dec induction were included in both studies, patients at MDACC received a 5-day Dec regimen in contrast to our patients, the majority of whom (63 of 83) received a 10-day decitabine (Dec10) induction. Despite these differences, Quintás-Cardama et al. [44] reported similar results as ours with higher response rates following IC but no statistically significant differences in OS following either IC or Epi induction in older patients.
At present, the “best” HMA regimen for upfront therapy of older AML patients has not yet been established. Two retrospective studies comparing the results of IC and Aza in unselected older individuals have been published. One demonstrated improved OS following IC; the other showed comparable survival for IC and Aza [27,42]. Here, we treated the majority of our Epi-treated patients (63/83) with a 10-day decitabine (Dec10) regimen based on prior reports of the tolerability, high response rates, and prolonged survival of patients on this regimen [19,51,52]. To address the potential efficacy of Dec10 as an induction strategy, we analyzed the outcomes of IC vs. Dec10 (n = 63), excluding those patients treated with Aza or 5-day Dec. Although our ORR (28%) following Dec10 was somewhat lower than the 31–47% previously reported, this may be attributed to our patients being older (all ≥ 60 years old), with higher rates of secondary or poor-risk karyotype disease [19,51,52]. Multivariate analysis accounting for the imbalanced clinical factors and the effect of HSCT in the two cohorts (IC vs. Dec10) again confirmed no significant differences in OS based on induction with 7 + 3 chemotherapy vs. Dec10. These data are the first to directly compare the results of a 10-day decitabine regimen with IC for older AML patients, albeit in a retrospective analysis.
In conclusion, our results suggest no meaningful benefit to intensive vs. epigenetic chemotherapy, specifically with a 10-day decitabine regimen, in AML patients aged 60 years and older. Although IC led to higher response rates, multivariate analyses accounting for differences in patient characteristics and HSCT demonstrated no statistically significant differences in OS or treatment-related mortality based on induction therapy. Given the baseline differences in the patients selected for each induction approach, however, we would endorse IC over HMA for induction of fit older patients with favorable/intermediate-risk karyotype, NPM-1 mutation, and hyperproliferative disease (as reflected by higher initial WBC, and increased peripheral or marrow blasts). In contrast, older individuals with poor-risk karyotype, wild-type NPM-1 mutation, secondary AML, and lower initial WBC may preferentially benefit from Epi therapy. Although not approved in the United States, the expanded off-label use of decitabine as an alternative induction approach for older AML patients has the potential to markedly increase the numbers (and outcomes) of individuals receiving definitive as opposed to supportive care only for this disease [41,42]. Epidemiological data from 2001 to 2009 have recently demonstrated a significant improvement both in percentage of older AML patients receiving treatment and the OS of patients as compared with 1992–2000 [41,53]. Although they may reflect better supportive care measures (i.e., antiemetics and azoles), these figures coincide with the increased availability and usage of HMA for AML therapy since 2005 [41]. The finding that decitabine induction may be more cost-effective than IC in some patients further supports its increased use [54]. Limitations of this study include its retrospective nature, small patient numbers (particularly in the subgroup analyses), treatment at a single academic medical center, and selection of induction regimen based on protocol eligibility and physician choice. A large multicenter trial prospectively randomizing older AML patients to upfront IC or 10-day decitabine therapy is underway to definitively address which subsets of patients are most likely to benefit from these different induction strategies.
Supplementary Material
Table I.
Baseline Characteristics of Older AML Patients by Induction Treatment Group
| Characteristic | Overall | IC | Epi | P |
|---|---|---|---|---|
| Patients, n (%) | 167 (100) | 84 (50) | 83 (50) | - |
| Male, n (%) | 117 (70) | 54 (64) | 63 (76) | 0.128 |
| Age, years (range) | 70 (60–92) | 67.5 (60–87) | 75.5 (60–92) | <0.001 |
| ECOG PS 0–1, n (%) | 139 (83) | 71 (85) | 68 (82) | 0.68 |
| Initial WBC count × 109/L, median (range) | 5.5 (0.3–238) | 12 (0–238) | 5 (0–121) | 0.03 |
| Hemoglobin, g/dL, median (range) | 9.3 (4.7–13) | 9 (6–13) | 9 (5–13) | 0.87 |
| Platelet count × 109/L, median (range) | 61 (3–622) | 69 (3–622) | 54 (10–501) | 0.94 |
| BM blasts (%), median (range) | 52 (4.2–96) | 53 (4–96) | 45 (10–96) | 0.18 |
| PB blasts (%), median (range) | 16 (0–96) | 21 (0–95) | 9 (0–96) | 0.06 |
| Secondary AML, n (%) | 82 (49) | 36 (43) | 46 (55) | 0.12 |
| Poor-risk cytogenetics, n (%) | 77 (47) | 23 (29) | 31 (40) | 0.2 |
| FLT-3 mutation, n (%) | 18 (11) | 12 (15) | 6 (7) | 0.21 |
| NPM-1 mutation, n (%) | 23 (15) | 15 (19) | 8 (10) | 0.17 |
| Subsequent HSCT, n (%) | 33 (20) | 26 (31) | 7 (8) | <0.001 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; WBC, white blood cell; BM, bone marrow; PB, peripheral blood; IC, intensive chemotherapy; Epi, epigenetic therapy.
Acknowledgments
The authors would like to thank Omar Al-Ustwani for helpful comments and feedback on the manuscript draft. In addition, we would like to acknowledge the outstanding care of our leukemia patients over the years by our clinical staffs (P. Doucette, J. Liu, J. Kwoka, M. Oldenberg, R. Sewastynowicz, L. Tiffany, S. Tighe, and L. Snopkowski).
Contract grant sponsor: Cancer Clinical Investigator Team Leadership Award (CCITLA) awarded by the National Cancer Institute; Contract grant number: P30CA016056 (to E.W.)
Contract grant sponsor: Roswell Park Alliance Foundation (Mr. and Mrs. E. LaFranier, the Jacquie Hirsch Leukemia Research Fund, and the Szefel Leukemia Research Fund) (to E.W.)
Contract grant sponsor: NCI Cancer Center Support Grant for RPCI; Contract grant number: CA016156.
Footnotes
Conflict of interest
The authors report no potential conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lofgren C, Albertioni F, Paul C. High activity and incomplete cross resistance of nucleoside analogues cladribine and fludarabine versus Ara-C on leukemic cells from patients with AML. Ther Drug Monit 2005;27:641–646. [DOI] [PubMed] [Google Scholar]
- 2.Estey E, Döhner H. Acute myeloid leukaemia. Lancet 2006;368:1894–1907. [DOI] [PubMed] [Google Scholar]
- 3.Latagliata R, Petti MC, Mandelli F. Acute myeloid leukemia in the elderly: ‘Per aspera ad astra’? Leuk Res 1999;23:603–613. [DOI] [PubMed] [Google Scholar]
- 4.Sekeres MA. Treatment of older adults with acute myeloid leukemia: State of the art and current perspectives. Haematologica 2008;93:1769–1772. [DOI] [PubMed] [Google Scholar]
- 5.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood 1999;94:1086–1099. [PubMed] [Google Scholar]
- 6.Büchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 2009;27:61–69. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006;106:1090–1098. [DOI] [PubMed] [Google Scholar]
- 8.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: The results of the United Kingdom Medical Research Council AML11 trial. Blood 2001;98: 1302–1311. [DOI] [PubMed] [Google Scholar]
- 9.Krug U, Buchner T, Berdel WE, et al. The treatment of elderly patients with acute myeloid leukemia. Dtsch Arztebl Int 2011;108:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschler B, de Witte T, Mertelsmann R, et al. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: Problems and approaches. Haematologica 2006;91:1513–1522. [PubMed] [Google Scholar]
- 11.Li KK, Luo LF, Shen Y, et al. DNA methyltransferases in hematologic malignancies. Semin Hematol 2013;50:48–60. [DOI] [PubMed] [Google Scholar]
- 12.Cang S, Lu Q, Ma Y, et al. Clinical advances in hypomethylating agents targeting epigenetic pathways. Curr Cancer Drug Targets 2010;10: 539–545. [DOI] [PubMed] [Google Scholar]
- 13.Leone G, D’Alò F, Zardo G, et al. Epigenetic treatment of myelodysplastic syndromes and acute myeloid leukemias. Curr Med Chem 2008; 15:1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC. Epigenetic therapy for elderly patients with AML. The Hematologist; May 1, 2012. [Google Scholar]
- 15.Oki Y, Issa JP. Epigenetic mechanisms in AML—A target for therapy. Cancer Treat Res 2010;145:19–40. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa ME, Lugthart S, Li Y, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 2010;17:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004;103:1635–1640. [DOI] [PubMed] [Google Scholar]
- 18.Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol 2010;28:556–561. [DOI] [PubMed] [Google Scholar]
- 19.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 2010;107:7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubbert M, Ruter BH, Claus R, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 2012;97:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010;28:562–569. [DOI] [PubMed] [Google Scholar]
- 22.Ramos F, Martinez-Robles V, Bargay J, et al. Azacitidine As Front-Line Therapy in AML: Results From Spanish National Registry. Alma Study Investigators. ASH Annual Meeting Abstracts 2012;120:3593. [Google Scholar]
- 23.Maurillo L, Venditti A, Spagnoli A, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: Report of 82 patients enrolled in an Italian Compassionate Program. Cancer 2012;118:1014–1022. [DOI] [PubMed] [Google Scholar]
- 24.Gavillet M, Noetzli J, Blum S, et al. Transfusion independence and survival in patients with acute myeloid leukemia treated with 5-azacytidine. Haematologica 2012;97:1929–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bories P, Bertoli S, Huguet F, et al. Efficacy of Frontline 5-Azacytidine in Older AML Patient Unfit for Chemotherapy. ASH Annual Meeting Abstracts 2011;118:2614. [Google Scholar]
- 26.Pleyer L, Stauder R, Burgstaller S, et al. Azacitidine in patients with WHO-defined AML—Results of 155 patients from the Austrian Azacitidine Registry of the AGMT-Study Group. J Hematol Oncol 2013;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Helm LH, Scheepers ER, Veeger NJ, et al. Azacitidine might be beneficial in a subgroup of older AML patients compared to intensive chemotherapy: A single centre retrospective study of 227 consecutive patients. J Hematol Oncol 2013;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thepot S, Itzykson R, Seegers V, et al. Azacitidine in untreated acute myeloid leukemia. A report on 149 patients. Am J Hematol 2014;89:410–416. [DOI] [PubMed] [Google Scholar]
- 29.Serrano J, Fuente Adl, Bergua J, et al. 5-Azacytidine versus intensive chemotherapy or BSC in elderly (>60 years) acute myeloid leukemia patients. A Retrospective Analysis. ASH Annual Meeting Abstracts 2011;118:2612. [Google Scholar]
- 30.Becker H, Suciu S, Rüter B, et al. Low-Dose Decitabine Vs Best Supportive Care In Older Patients With AML and Low Blast Counts: Results Of a Subgroup Analysis Of The Randomized Phase III Study 06011 Of The EORTC Leukemia Cooperative Group and German MDS Study Group. Blood 2013;122:1452. [Google Scholar]
- 31.Kaminskas E, Farrell AT, Wang YC, et al. FDA drug approval summary: Azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist 2005;10:176–182. [DOI] [PubMed] [Google Scholar]
- 32.FDA approval for 5-day outpatient dosing for Dacogen for MDS. Oncol Times 2010;32:42. doi: 10.1097/1001.COT.0000370083.0000372821.0000370049 [DOI] [Google Scholar]
- 33.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes:Results of a phase III randomized study. Cancer 2006;106:1794–1803. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21: 4642–4649. [DOI] [PubMed] [Google Scholar]
- 36.JP F, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 37.Latouche A, Allignol A, Beyersmann J, et al. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013;66:648–653. [DOI] [PubMed] [Google Scholar]
- 38.Cox DR. Regression models and life tables. J R Stat Soc Ser B 1972;34:187–220. [Google Scholar]
- 39.Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 651 years with acute myeloid leukemia: A randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol 1989;7:1268–1274. [DOI] [PubMed] [Google Scholar]
- 40.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009;113: 4179–4187. [DOI] [PubMed] [Google Scholar]
- 41.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012;97:1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bories P, Bertoli S, Bérard E, et al. Intensive chemotherapy, azacitidine or supportive care in older acute myeloid leukemia patients: An analysis from a regional healthcare network. Am J Hematol 2014;89:E244–E252. [DOI] [PubMed] [Google Scholar]
- 43.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 2010;116:4422–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120:4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behringer B, Pitako JA, Kunzmann R, et al. Prognosis of older patients with acute myeloid leukemia receiving either induction or noncurative treatment: A single-center retrospective study. Ann Hematol 2003;82:381–389. [DOI] [PubMed] [Google Scholar]
- 46.Neuss MN, Feussner JR, DeLong ER, et al. A quantitative analysis of palliative care decisions in acute nonlymphocytic leukemia. J Am Geriatr Soc 1987;35:125–131. [DOI] [PubMed] [Google Scholar]
- 47.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol 2009;145:598–605. [DOI] [PubMed] [Google Scholar]
- 48.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol 2014;32:2541–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daver N, Liu Dumlao T, Ravandi F, et al. Effect of NPM1 and FLT3 mutations on the outcomes of elderly patients with acute myeloid leukemia receiving standard chemotherapy. Clin Lymphoma Myeloma Leuk 2013;13:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J Clin Oncol 2010;28:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma 2013;54:2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma 2014;55:1533–1537. [DOI] [PubMed] [Google Scholar]
- 53.Shah BK, Ghimire KB. Improved survival among older acute myeloid leukemia patients—A population-based study. Acta Oncol 2014;53: 935–938. [DOI] [PubMed] [Google Scholar]
- 54.Batty N, Yin Y, Wetzler M. Decitabine is more cost effective than cytarabine and daunorubicin in elderly acute myeloid leukemia patients. J Cancer Res Ther 2014;2:68–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.