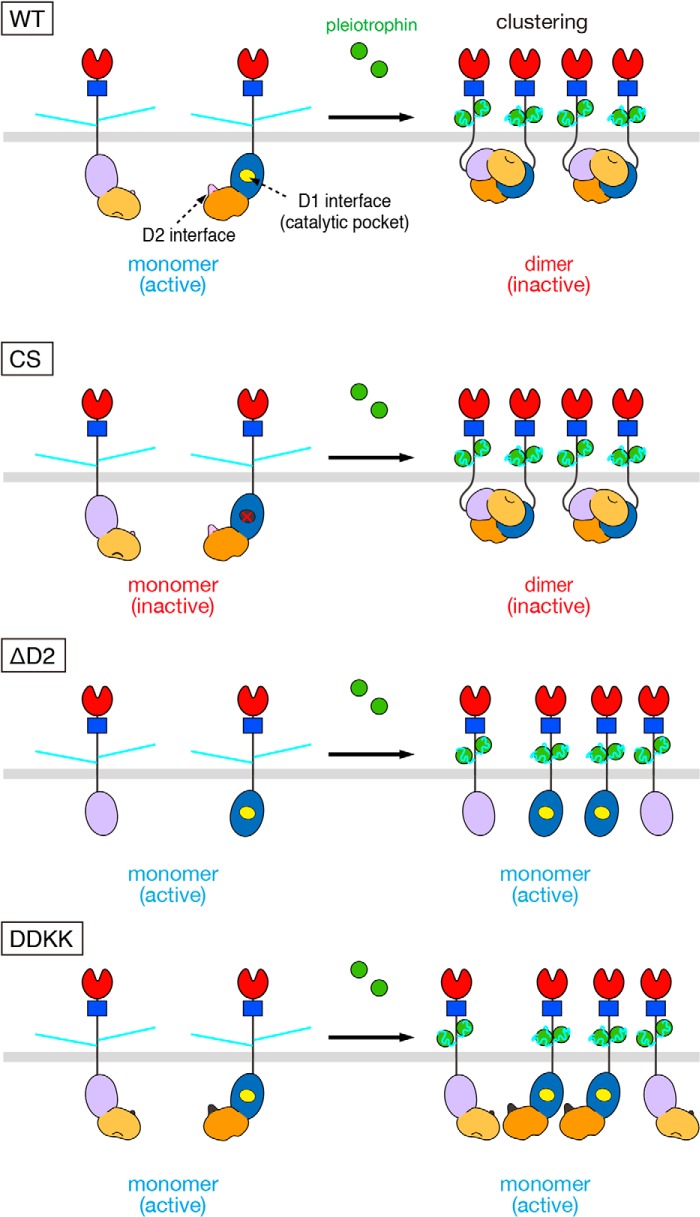
Figure 5.
Models for ligand-induced clustering and dimerization for WT and mutant PTPRZ-B. PTPRZ-B receptors are expected to exist as monomers, but undergo clustering by extracellular ligand (PTN) binding, facilitating the head-to-toe dimer formation of the intracellular part, thereby masking the catalytic site of D1 by D2 from another (WT). On the other hand, the Cys-1930 to Ser mutation (CS) results in inactivation of the D1 domain but has no effects on the ligand-induced dimerization. The loss of the regulatory D2 domain (ΔD2) and the mutation in the dimer interface on D2 (DDKK) are catalytically active, but lacking the ligand-induced PTPase inactivation; however, ΔD2 and DDKK mutants are sensitive to PTPase inhibitors that bind to the active site. Domains are highlighted in different colors: carbonic anhydrase-like (red), fibronectin type III (FNIII, blue), PTP-D1 (blue or light purple), and PTP-D2 (orange or yellow-orange) domains. The extracellular regions of the three isoforms are highly glycosylated with chondroitin sulfate chains (light blue lines).