Figure 1.
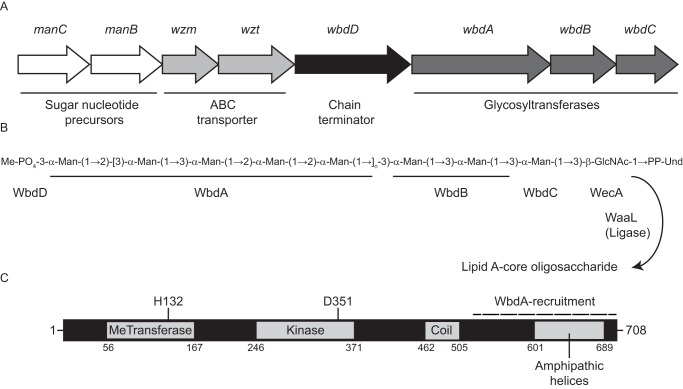
Structure and biosynthesis of the E. coli O9a O-PS. A, organization of the O-PS synthesis (wb*) operon. B, structure of the Und-PP–linked O9a biosynthetic intermediate. The square brackets delineate the tetrasaccharide O-PS repeat unit. Man, d-mannose; GlcNAc, d-GlcNAc. C, domain organization of the dual kinase methyltransferase WbdD. The coiled-coil domain acts as a molecular ruler separating WbdA, which is recruited to its interaction site at the C terminus of WbdD (indicated), from the chain-terminating catalytic sites of WbdD.