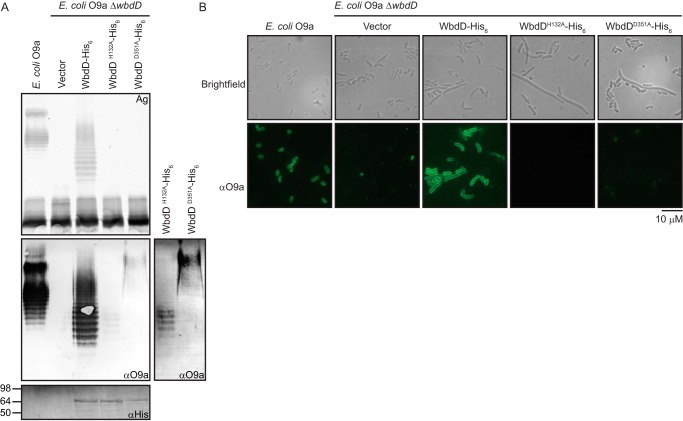
Figure 3.
The complete O-antigen–terminating moiety is essential for export of O9a O-PS and cell-surface exposure. E. coli O9a manA ΔwbdD (CWG900) cells were transformed with plasmids expressing WbdD-His6 (pWQ470) and its variants, WbdDH132A-His6 (pWQ829) or WbdDD351A-His6 (pWQ830), and examined by SDS-PAGE and immunoblotting (A) or immunofluorescence microscopy (B). Protein expression was induced with 0.02% l-arabinose. E. coli O9a manA (CWG634) provided the positive control. A, the top panel displays the LPS profile of proteinase K–treated whole-cell lysates resolved by SDS-PAGE and stained with silver. The middle panel is an immunoblot using polyclonal primary antibodies directed against O9a (note that immunoblotting detects both Und-PP-linked and lipid A-linked O-PS). Due to the high signal from complete LPS compared with Und-PP–linked intermediates in equivalent amounts of cells, a longer exposure of the lanes containing the Und-PP–linked intermediates is shown on the right. The bottom panel is a Western immunoblot using antibodies directed against the C-terminal His6 tags on WbdD to confirm protein expression. B, immunofluorescence microscopy of the corresponding cells, using O9a-specific antibodies to detect surface-exposed O9a O-PS. Brightfield and immunofluorescence microscopy experiments were performed on formalin-fixed whole cells, probed with an anti-O9a rabbit antiserum and an FITC-conjugated secondary antibody.