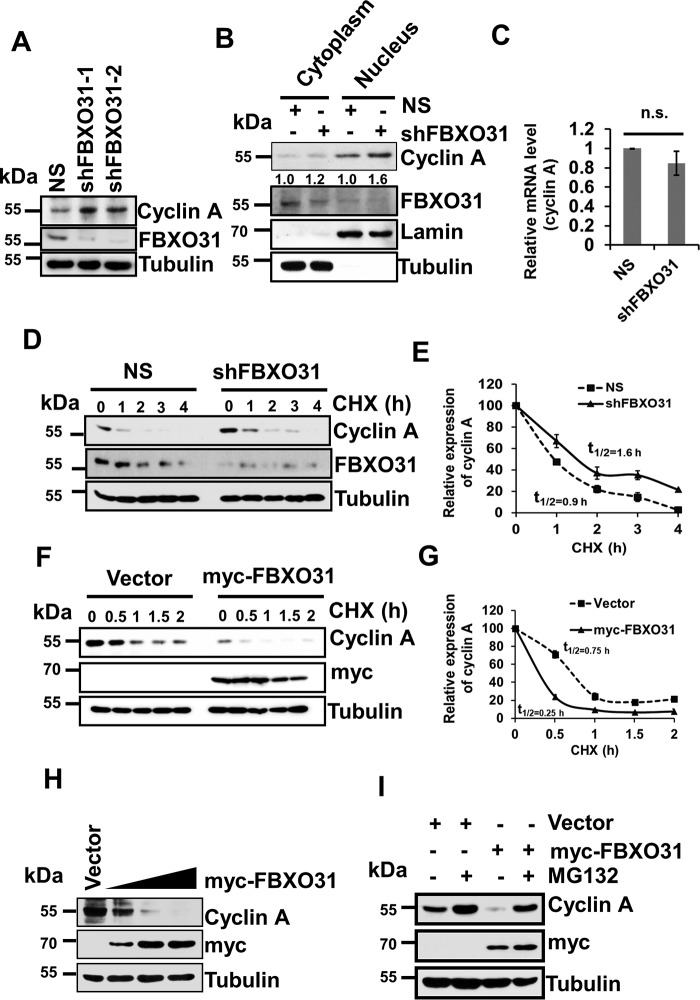
Figure 2.
FBXO31 regulates cyclin A expression at the proteasomal level. A, immunoblot monitoring the level of cyclin A and FBXO31 in MCF7 cells expressing either NS or FBXO31 shRNAs. Whole-cell protein extracts were immunoblotted to probe for the indicated proteins. B, cytoplasmic and nuclear fractions of cells expressing either NS or FBXO31 shRNA were immunoblotted with the indicated antibodies. Tubulin and Lamin B1 were used as cytoplasmic and nuclear loading controls, respectively. C, real time RT-PCR was performed to monitor cyclin A mRNA expression in NS and shFBXO31 cells. Cyclin A mRNA level was normalized to the GAPDH mRNA level. Error bars represent S.E. from three independent experiments. D, immunoblots monitoring the turnover profile of cyclin A in NS and shFBXO31 cells following cycloheximide (CHX) (40 μg/ml) chase for the indicated time periods. E, quantification of levels of cyclin A in D. Expression of cyclin A was normalized with tubulin, and then cyclin A expression at 0 h was considered as 100% relative to the values of other time points that were plotted. F, immunoblot monitoring the turnover of cyclin A in cells expressing either empty vector or myc-FBXO31 following cycloheximide (40 μg/ml) chase for the indicated periods. Cells were transfected with the indicated constructs for 36 h and following that whole-cell protein extracts were immunoblotted to probe for the indicated proteins. G, quantitation of half-life of cyclin A from F. Expression of cyclin A was normalized with tubulin, and the degradation profile was plotted considering the expression of cyclin A at 0 h as 100%. H, cells were transfected either with empty vector or increasing concentrations of myc-FBXO31 for 48 h. Cells were then harvested, and whole-cell protein lysates were immunoblotted to probe for indicated proteins. I, cells were transfected with either empty vector or myc-FBXO31 for 36 h and were grown in the presence or absence of 10 μm proteasome inhibitor MG132 for 6 h and were immunoblotted and probed with the indicated antibodies. Error bars where shown represent S.E. from three independent experiments, and n.s. represents nonsignificant; *, p ≤ 0.05; **, p < 0.01; ***, p < 0.001.