Table 1.
Development of the Catalytic Formal Ene Reaction
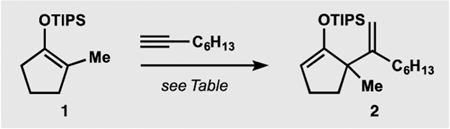 | |||||
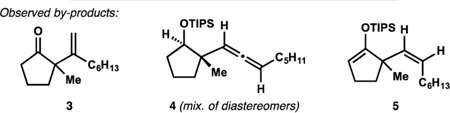 | |||||
| Entry | Conditionsa | 2(%)b | 3(%)b | 4(%)b,c | 5(%)b |
| 1 | 5 mol%IPrAuCI,5 mol% NaBArF, 65℃ | 11 | 8 | 10 | <1 |
| 2 | 10 mol% ZnBr2, 65℃ | 4 | 5 | 1 | <1 |
| 3 | 5 mol% In(OTf)3, 65℃ | <1 | 1 | <1 | <1 |
| 4 | 5 mol% InCI3, 65℃ | 5 | 9 | <1 | <1 |
| 5 | 5 mol% InBr3, 65℃ | 72 | 5 | 6 | 2 |
| 6 | 5 mol% InI3, 65℃ | 36 | 6 | 7 | <1 |
| 7 | 5 mol% InBr3, 5 mol% NaBArF,65℃ | 40 | 11 | 3 | <1 |
| 8d | 5 mol% InBr3,50℃ | 65 | 9 | 3 | <1 |
| 9 | 5 mol% MeOH, 8 mol% TMSBr, 65℃ | <1 | <1 | <1 | <1 |
| 10 | 5 mol% TfOH, 65℃ | <1 | <1 | <1 | <1 |
| Attempted alkenylation of silyl enol ether 6: | |||||
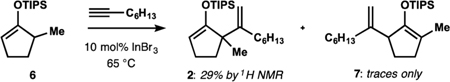 | |||||
0.25–0.5 mmol of 1, 1.5 equiv of alkyne, 0.25–0.5 mL of (CH2Cl)2, 20–28 h.
Based on internal standard and determined by 1H NMR.
dr = 1:1.
65% isolated yield of 2.
