Table 2.
Preliminary Substrate Scope of the Alkenylationa
 |
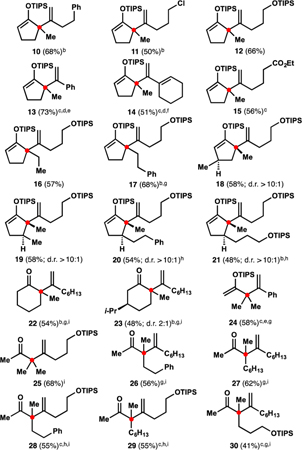 |
Typically, starting silyl enol ethers were mixtures of isomers; see SI for details. 1 equiv of silyl enol ether, 1.5 equiv of alkyne, 10 mol% of InBr3, (CH2Cl)2 (1 M in enoxysilane), 65 °C, ca. 24 h; rr ≥ 10:1 (except 15, rr 9:1; 24, rr 7:1). Siloxydienes (except 13, 14, and 24) contained ca. 5 mol% of inseparable allenes.
Heated to 50 °C.
Heated to 80 °C.
5 mol% of InBr3.
Neat. f5 M in silyl enol ether.
Heated for ca. 72 h.
Heated for ca. 48 h.
After hydrolysis.
