Figure 1.
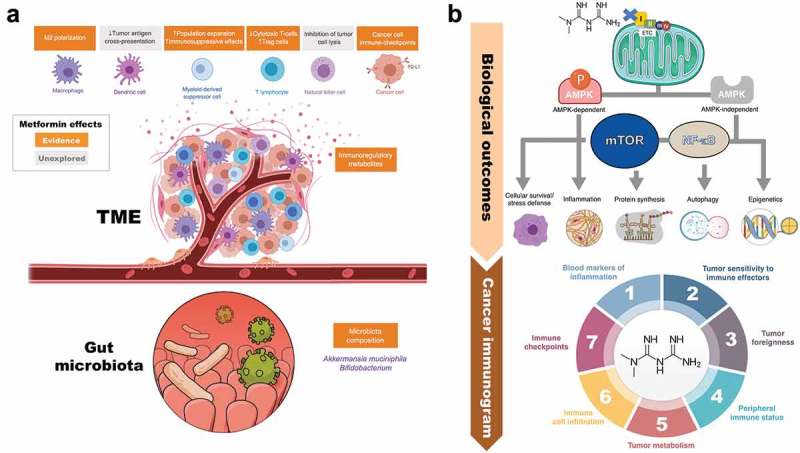
Metformin: A multi-faceted immuno-metabolic adjuvant for cancer immunotherapy. (a). Evidences. The anti-diabetic agent metformin might serve as an archetype immuno-metabolic adjuvant capable of simultaneously regulating, in the appropriate direction and intensity, antitumor immunity-related metabolic checkpoints not only in T-cells, cancer cells and associated immune suppressor cells of the TME, but also in the gut microbiota and its systemic effects on host metabolism. The capacity to improve the metabolic competence of T-cell immune surveillance, suppress the metabolic traits of immunosuppressive cell subsets in the TME, prevent both the constitutive and the inflammation (IFNγ)-inducible expression of immune checkpoint receptors in cancer cells, and shift the gut microbiota composition towards specific commensal microbes might optimize the effectiveness of cancer immunotherapy. Further studies are needed to determine the effects of metformin on tumor antigen cross-presentation by dendritic cells and tumor cell lysis by natural killer cells. (b). Mechanisms. As a consequence of the metformin-mediated inhibition of mitochondrial electron transfer, metformin is able to activate a variety of AMPK-dependent and -independent signaling pathways through which it facilitates the inhibition of mTOR, inhibits the inflammatory pathway, and lastly disturbs inflammation, cellular survival, stress defense, protein synthesis, autophagy, and epigenetic reprogramming .13–20 Downstream of these major biological outcomes, metformin might have the capacity to impact all the cancer-immune system interactions constituting the so-called “cancer immunogram”.21 Metformin might lead to systemically decreased levels of pro-inflammatory soluble inhibitors (e.g., serum levels of C-reactive protein and IL-622–24), which are known to drive tumor-associated inflammation, impair T cell-mediated tumor control, and associate with poor outcomes in response to ICIs (1). Metformin might increase tumor sensitivity to immune effectors by augmenting the levels of major histocompatibility complex (MHC) class I antigens25 (2), which might impact also tumor foreignness by helping T-cells to recognize neoantigens (3). Metformin might alter the general performance of immune system via modification of the microbiome26 (4), specifically by changing microbial folate and serine/methionine metabolism.27–29 Metformin might reverse an inhibitory tumor metabolism by remodeling the hypoxic TME via reduction of intratumoral hypoxia (5), a key driver of poor outcomes upon ICIs. Metformin might sustain or restore the infiltration of tumor-reactive T-cells into the tumor (6) by preventing the occurrence of dysfunctional states characterized by impaired activity and proliferative activity, increase apoptotic rate, and reduced production of effector cytokines (i.e., T-cell exhaustion). Metformin might alter the expression profile of immune checkpoints (7) such as PD-L1 in the tumor compartment [37, Figure 2], thus suggesting that a combination of metformin-CTLA-4 blockade might have the potential to increase the efficacy of cancer immunotherapy.
