Table 1.
Epigenetic compounds approved by Food and Drug Administration (FDA), treatment indication and epigenetic mechanism of action.
| FDA approved Drug (commercial name) |
Year | Treatment | Mechanism of action | Structural formula |
|---|---|---|---|---|
| Azacitidine+ decitabine or low-dose cytarabine (Venclexta) |
2018 | Acute myeloid leukemia | DNTMi | 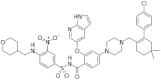 |
| Panobinostat + Bortezomib + Dexamethasone (Farydak) |
2015 | Multiple myeloma | HDACi | 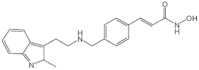 |
| Belinostat (Belodaq) |
2014 | Peripheral T Cells Lymphoma | HDACi | 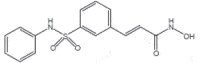 |
| Valproic Acid | 2010 | Antidepressive Neurologic disorders |
HDACi | 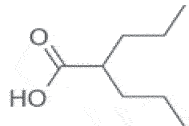 |
| Romidepsin (Ixodax) |
2009 | Cutaneous T-cell lymphoma | HDACi | 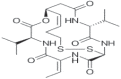 |
| 5 Azacitidine (Vidaza) |
2009 | Myelodysplastic syndrome | DNTMi | 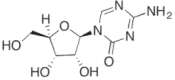 |
| Vorinostat (Zolinza) |
2006 | Cutaneous T-cell lymphoma | HDACi | 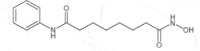 |
DNMTi, DNA methyltransferase inhibitor; HDACi, Histone desacetilase inhibitor
