Figure 2.
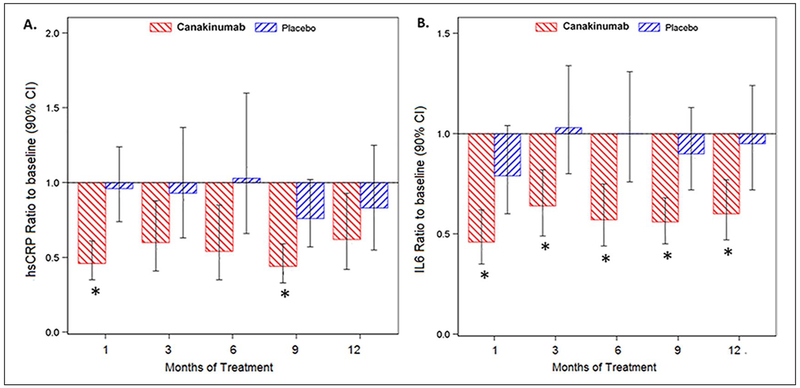
Canakinumab treatment leads to an overall decrease in inflammatory biomarkers. Patients were treated monthly with 150 mg canakinumab or placebo sc. There was a decrease (shown here as ratio to baseline) in hsCRP (A) and IL-6 (B) seen as early as 1 month of treatment that appears durable throughout the treatment period compared to placebo.
*p < 0.05 vs placebo.
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; sc, subcutaneously.
