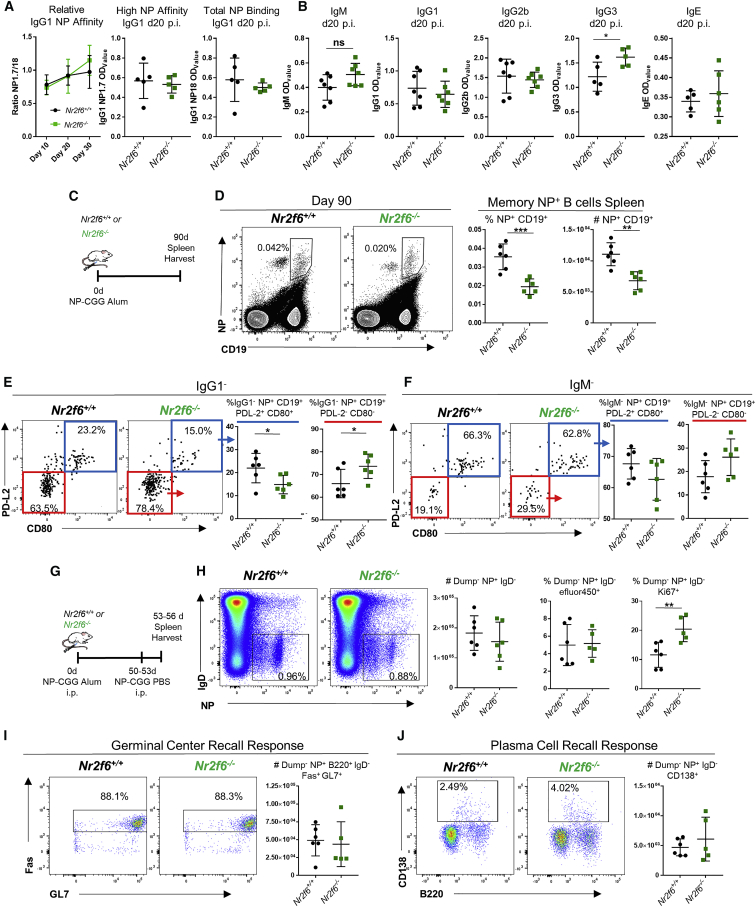
Figure 2.
Nr2f6 Deficiency Does Not Alter Affinity Maturation but Affects Antigen-Specific Memory B Cells
(A) Nr2f6+/+ or Nr2f6−/− mice were immunized with NP-CGG in alum; relative binding of high (18) or low (1.7) ratio haptenated NP-BSA-coated plates was measured by ELISA. IgG1 relative affinity is shown from serum collected 10, 20, or 30 days after immunization. High-affinity (NP1.7) or total IgG1 binding (NP18) titers from one representative ELISA experiment are shown.
(B) Sera was collected, and ELISA was performed for indicated antibodies 20 days after immunization, optical density 50 (OD50) is shown.
(C) Experimental scheme.
(D) Nr2f6+/+ or Nr2f6−/− mice were immunized with NP-CGG, and spleens were harvested on day 90; antigen-specific memory B cells were stained with NP-PE and CD19. Representative flow cytometry plots are shown in the left two panels, frequency and total cell count of this population are shown in the right two panels.
(E–G) CD80 and PD-L2 were investigated by flow cytometry on IgG1− (E) or IgM− (F) antigen-specific memory B cells from both Nr2f6+/+ or Nr2f6−/− genotypes. Recall responses were tested as described in the scheme (G).
(H) Total NP-specific, IgD−, and dump gate negative (Gr-1, F4/80, CD4, and CD8) cells, proliferation, and dead cell frequency were investigated by Ki67 and efluor450 fixable dye staining.
(I and J) GC (I) and PC (J) responses from this population were investigated by flow cytometry. Representative flow cytometry plots and total counts are shown.
Memory results are representative of two independent experiments with n ≥ 5. The middle bar represents the dataset average. Error bars represent SD, and an asterisk indicates statistically significant differences calculated using two-tailed Student’s t test. A p value of <0.05 was considered statistically significant. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.