Figure 1. E2F7 and E2F8 are subjected to Cullin‐RING ligase‐dependent degradation during G2 and early M phase.
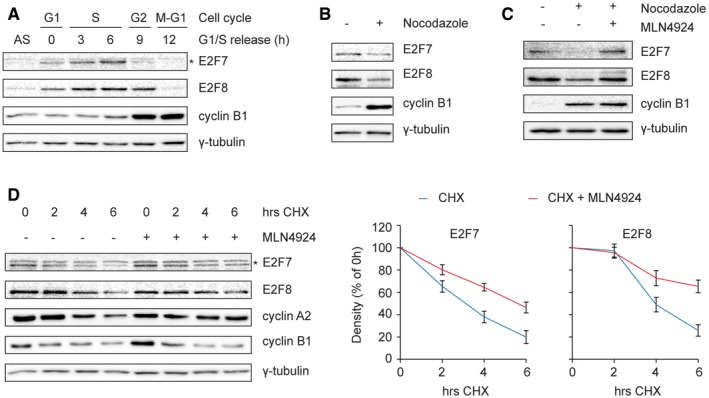
- Protein levels of E2F7 and E2F8 during cell cycle progression. HeLa cells were synchronized by a double thymidine block and released into fresh medium. Cells were harvested at the indicated time points, and an asynchronous (AS) condition was used as control. Protein levels were measured by immunoblotting, and cell cycle progression was determined by flow cytometry (shown in Fig EV1A). The asterisk indicates the E2F7‐specific band.
- Decreased stability of E2F7 and E2F8 in nocodazole‐arrested cells. HeLa cells were treated with either DMSO or nocodazole (50 ng/ml) for 16 h. Cells were harvested and lysed for immunoblotting. Protein expression of cyclin B1 was used as a marker for G2 or M, and γ‐tubulin was used as loading control.
- Selective Cullin‐RING inhibitor MLN4924 rescued the degradation of E2F7/8 under nocodazole‐arrested condition. HeLa cells were treated with DMSO, nocodazole, or nocodazole plus MLN4924 (0.1 μM) for 16 h. Cells were harvested and lysed for immunoblotting. Cyclin B1 expression was used as a marker for G2 or M cell cycle progression, and γ‐tubulin was used as loading control.
- Increased half‐life of E2F7/8 by MLN4924 treatment. HeLa cells were treated with cycloheximide (CHX, 50 μg/ml) either with or without MLN4924 (0.1 μM). Protein levels of E2F7 and E2F8 were determined by immunoblotting (left panel). Asterisk indicates the E2F7‐specific band. Cyclin B1 and cyclin A2 expressions were used as a marker for G2 or M cell cycle progression, and γ‐tubulin was used as loading control. Quantifications (right panels) were performed based on two independent experiments. Bar and error bars represent mean ± SEM.
Source data are available online for this figure.
