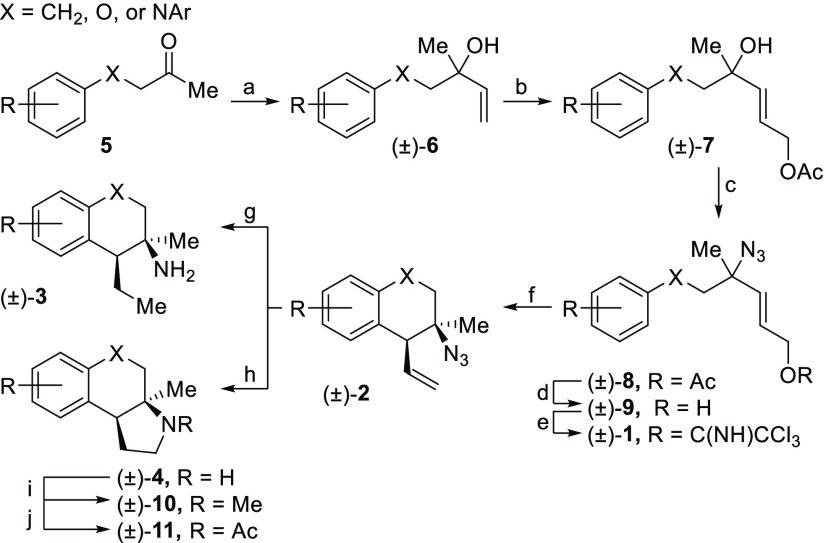
Scheme 2. Synthesis of (±)-GPCR Ligands.
Reagents and conditions: (a) vinyl MgCl, THF, 0 °C, 30 min, 83–91%; (b) cis-1,4-diacetoxy-2-butene, 1–2 mol % Hoveyda–Grubbs second generation catalyst, 40 °C, 18 h, 68–86%; (c) TMSN3, 10 mol % Zn(OTf)2, rt, 90 min, 29–87%; (d) K2CO3, MeOH, rt, 30 min, 97%–quant.; (e) NCCCl3, 20 mol % DBU, rt, 90 min, 73–97%; (f) 10 mol % AgSbF6, CHCl3, 40–60 °C, 24 h, 39–94%; (g) H2, 10% w/w Pd/C, MeOH, 18 h, 52%-quant; (h) HBCy2, DCM, 0 °C to rt, 18 h, 35–84%; (i) aq. CH2O, NaBH3CN, HOAc, NCCH3, 0 °C to rt, 30 min, 70–89%; (j) Ac2O, TEA, DMAP, 0 °C to rt, 18 h 73–96%.