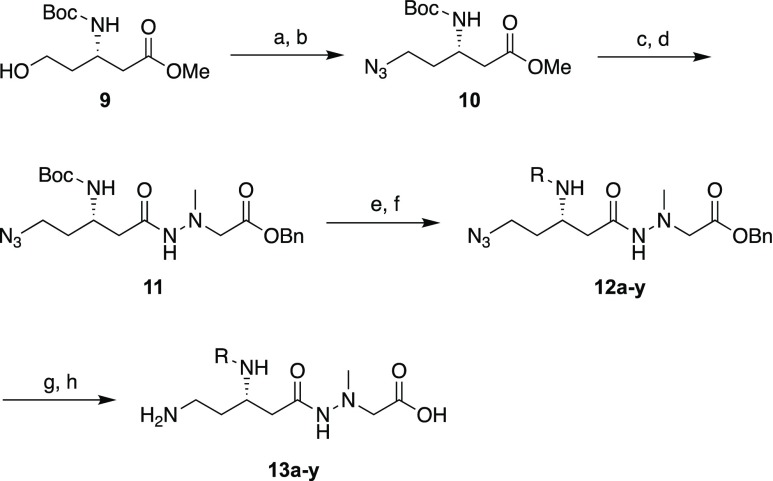
Scheme 1. Synthesis of Derivatives 13a–y.
Reagents and conditions: (a) MsCl, Et3N, CH2Cl2, RT, overnight, 90%. (b) NaN3, DMF, RT, overnight, 91%. (c) KOH, MeOH/H2O, 5 h. (d) H2NN(Me)CH2CO2Bn, EDC·HCl, HOBt·H2O, Et3N, DMF, RT, overnight, 81% (2 steps). (e) 4 M HCl/dioxane, RT, 1 h. (f) Protected amino acid or 4-methylpentanoic acid, EDC·HCl, HOBt·H2O, Et3N, DMF, RT, overnight, 49–98% (2 steps). For 13d, (g) H2, Pd/C, MeOH, RT, 1 h, then RP-HPLC, 27% (2 steps). For 13a–c and 13e–y, (h) H2, Pd/C, MeOH, RT, 1 h, then, 4 M HCl/dioxane, RT, 1 h, then RP-HPLC, 7–64% (3 steps).