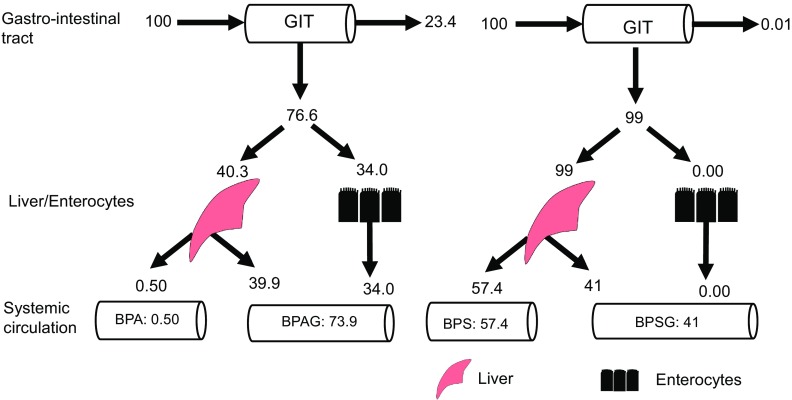
Figure 4.
Summary of the main results in terms of the fate of a dose of BPA and BPS administered by orogastric gavage. Note: The estimated absorption of BPA by the enterocytes was 76.6%, 23.4% of the BPA not being absorbed, whereas BPS absorption was near total (99%). Most of the BPA dose was subjected to intestinal (34%) and hepatic (40%) metabolism, resulting in a low oral BPA bioavailable dose (0.50%). The fraction of BPA undergoing an intestinal first-pass effect gained access to the blood and contributed to the systemic exposure to BPAG that accounted for 73.9% of the dose. By contrast, only 41% of the BPS oral dose reaching the portal blood was metabolized by the liver and contributed to the systemic exposure to BPSG (41%), with 57.4% of the BPS oral dose being bioavailable. The numbers represent the bootstrap estimates of the median, which explains why the sum is not 100. BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; GIT, Gastrointestinal tract.