Abstract
Background:
Long-term exposure to particulate matter (PM) air pollution is associated with all-cause mortality and adverse cognitive outcomes, but the association with developing depression remains inconsistent.
Objective:
Our goal was to evaluate the prospective association between PM air pollution and developing depression assessed using the Center for Epidemiological Studies Depression (CES-D) scale.
Methods:
Subjects were drawn from a prospective cohort study of 123,045 men and women free of depressive symptoms at baseline who attended regular screening exams in Seoul and Suwon, South Korea, from 2011 to 2015. Exposure to PM with an aerodynamic diameter of ( and , respectively) was estimated using a land-use regression model based on each subject’s residential postal code. Incident depression was defined as a CES-D score during follow-up. As a sensitivity analyses, we defined incident depression using self-reports of doctor’s diagnoses or use of antidepressant medications during follow-up.
Results:
The mean baseline 12-month concentrations of and were 50.6 (4.5) and , respectively. The hazard ratios (HRs) and 95% confidence intervals (CIs) for developing depression associated with a increase in 12- and 60-month exposure were 1.11 (95% CI: 1.06, 1.16) and 1.06 (95% CI: 1.01, 1.11), respectively. The corresponding HRs for 12-month exposure was 0.96 (95% CI: 0.64, 1.43). Similar results were obtained when incident depression was identified using self-reports of doctor’s diagnoses or the use of antidepressant medications.
Conclusion:
In this large cohort study, we found a positive association between long-term exposure to outdoor air pollution and the developing depression. We did not find an association for outdoor air pollution; however, we had a much shorter follow-up for subjects’ exposure to . https://doi.org/10.1289/EHP4094
Introduction
Long-term exposure to particulate matter (PM) air pollution is associated with all-cause mortality and with several chronic diseases, including respiratory and cardiovascular disease (Ailshire and Crimmins 2014; Di et al. 2017; Hart et al. 2015b; Zhang et al. 2016). Rapidly accumulating evidence also suggests that air pollution may be associated with depression, a very common mental disorder with a substantial impact on the quality of life, morbidity, mortality, and health care expenditures (James et al. 2017; Simon 2003; WHO 2017).
The association between air pollution and depression, however, is inconsistent across studies (Kim et al. 2016; Kioumourtzoglou et al. 2017; Lin et al. 2017; Pun et al. 2017; Szyszkowicz et al. 2016; Vert et al. 2017; Wang et al. 2014; Zijlema et al. 2016). Prior studies often identified incident depression based on doctors’ diagnosis or on the use of antidepressant medications and may have missed a high proportion of subjects who did not realize they had depressive symptoms or who did not seek medical treatment (Pratt and Brody 2014). Because a high proportion of subjects with depressive symptoms do not seek medical care (Bell et al. 2010, 2011; Wong et al. 2012), this type of misclassification may bias the observed association between air pollution and depression. Although the CES-D scale is not a gold standard for diagnosing depression, it is a validated reliable screening instrument used extensively in population studies that can improve detection of depression cases, particularly of mild and moderate cases. Previous studies based on CES-D scores have found an inconsistent association between long-term air pollution exposure and depressive symptoms, but their sample size was small and they had limited power to identify an association (Pun et al. 2017; Wang et al. 2014; Zijlema et al. 2016). Additional reasons for inconsistent associations across studies include variations in air pollution levels and differences in study settings, demographic characteristics, and preexisting conditions of study populations.
To address the limitations of prior studies, we evaluated the prospective association between PM air pollution and depression assessed using the CES-D score as well as reported diagnoses and medications use in a large cohort of Korean men and women who attended repeated health screening exams between 2011 and 2015. We hypothesized that subjects with higher long-term exposure to particles () and in aerodynamic diameter () would be at higher risk of developing depression over follow-up (indicated by a CES-D score ) compared with subjects exposed to lower concentrations of and .
Methods
Study Population
The Kangbuk Samsung Health Study (KSHS) is an ongoing cohort study of South Korean men and women of age or older who underwent a comprehensive annual or biennial health examination at the clinics of the Kangbuk Samsung Hospital Total Healthcare Center in Seoul and Suwon, South Korea (Kim et al. 2018). In South Korea, the Industrial Safety and Health Law requires that employees undergo annual or biennial health screening exams covered by employers. In our study, over 80% of the subjects were employees and/or their spouses of various companies or local government organizations. The remaining subjects voluntarily purchased screening exams at the health exam center. The study was approved by the institutional review board of the Kangbuk Samsung Hospital, and the requirement of informed consent was waived because we only used deidentified data routinely collected during health screening visits.
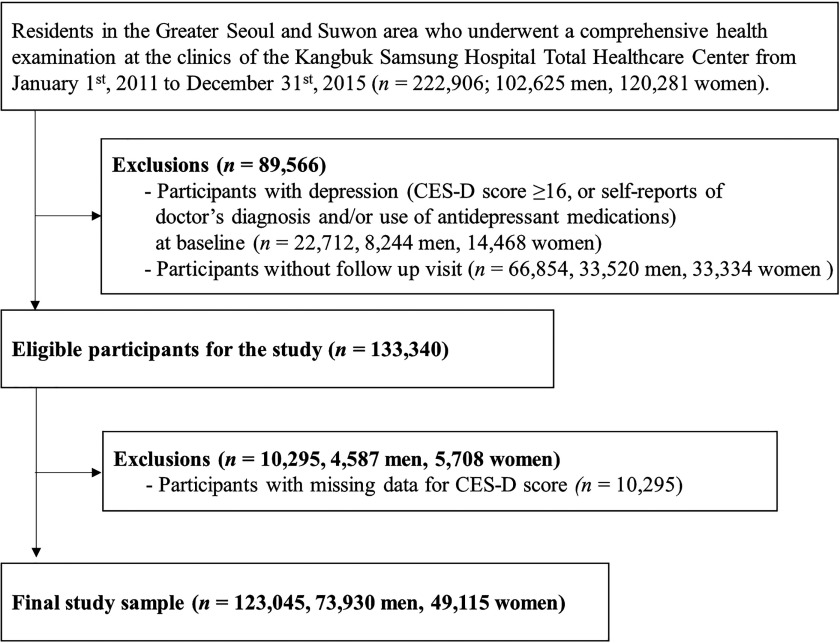
In this analysis, we used data from subjects who lived in the Greater Seoul and Suwon metropolitan areas and who underwent a comprehensive health examination from 1 January 2011 through 31 December 2015 (; Figure 1). We excluded subjects with depression at baseline (self-reports of doctor’s diagnosis and/or use of antidepressant medications or CES-D score ; ; 8,244 men, 14,468 women), subjects without any follow-up visit (; 33,520 men, 33,334 women), and subjects with missing data for CES-D scores (; 4,587 men, 5,708 women). The final sample size was 123,045 (73,930 men, 49,115 women). The study was approved by the institutional review board of the Kangbuk Samsung Hospital, and the requirement of informed consent was waived because we only used deidentified data routinely collected during health screening visits.
Figure 1.
Flowchart of study subjects.
Assessment of PM Air Pollution
We estimated monthly and concentrations for each individual subject using a land-use regression model based on subjects’ address postal codes (Yanosky et al. 2014; Zhang et al. 2018). Briefly, we used air pollution monitoring data from the Seoul Metropolitan Government Atmospheric Environment, the Gyeonggi-do Institute of Health and Environment, and the Korean National Climate Data Service System (NCDSS). data were available from January 2002, whereas data were available from January 2015. We estimated monthly mean PM concentrations for each subject using a mixed generalized additive regression model including smoothed county population density, altitude, distance to point-source emission (power plants), percentage of land cover within 300-, 500-, 1,000-, 3,000-, and buffers, and distance to the nearest road. For each subject, we then calculated the 12- and 60-month mean concentration and 12-month mean concentration prior to each visit. The models were evaluated by several cross-validation approaches, including the holdout method, K-fold cross-validation, and leave-one-out cross-validation (Yanosky et al. 2014). Holdout cross-validation results demonstrated that the models had a high predictive accuracy (cross-validation values of 0.80 and 0.77 for and , respectively).
Health Screening Exams
At each screening exam, trained nurses used standardized questionnaires to collect information on sociodemographic factors, lifestyle characteristics, disease history, and medication use (Jeon et al. 2019; Kim et al. 2018). Education level was categorized as no education, elementary school, middle school, high school, technical college, and university or more. Smoking status was categorized as never, former, current, and unknown. Frequency of alcohol consumption was categorized as none, moderate drinking (men: , women: ), excessive drinking (men: , women ), and unknown. We categorized physical activity levels using the validated Korean version of the International Physical Activity Questionnaire (IPAQ) Short Form (Chun 2012). The IPAQ Short Form measures the frequency and duration of walking or any other moderate-to-vigorous physical activity undertaken for more than 10 continuous min across all contexts (i.e., work, home, and leisure) during a 7-day period. The physical activity levels were classified into three categories: none, , , and unknown. Height and weight were measured and body mass index (BMI) was calculated as weight in kilograms divided by height squared. BMI was categorized as underweight (), normal weight (), overweight (), obese (), and unknown, following specific cutoffs for Asian populations. Missing data in covariates were considered as a separate category in statistical analyses.
CES-D Scale
At each screening examination, subjects completed a self-administered questionnaire that included the Korean version of the CES-D scale (Kim et al. 2007). The scale comprises 20 items that evaluate different moods or behaviors related to depression, with each item rated from 0 (rarely or none of the time) to 3 (most or almost all the time). CES-D scores range from 0 to 60, with higher scores indicating a higher frequency of depressive symptoms. A CES-D score is usually considered as a threshold to trigger a visit to a professional counselor (Radloff 1977).
Incident outcome events (defined either as CES-D or as a doctor’s diagnosis or medication use) were identified at the follow-up visits. Follow-up thus extended from the visit in which an incident outcome event was first identified (in cases) or otherwise until the last follow-up visit available (in noncases). The follow-up period for exposure was from 1 January 2011 to 31 December 2015; the follow-up period for exposure was from 1 January 2015 to 31 December 2015.
In sensitivity analyses, we used only a doctor’s diagnosis and/or use of antidepressant medications to identify incident cases of depression. In this analysis, conducted in the same population as the primary analyses, subjects with CES-D score but without a report of a doctor’s diagnosis or antidepressant medications use were not identified as cases. As a second sensitivity analysis, we conducted analyses for exposure with data restricted to the same time frame as analyses. We also conducted analyses for exposure using the same group of subjects to but with follow-up starting in 2011.
Statistical Analysis
We used flexible parametric proportional hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of depression associated with a increase in and . The exposure was the mean 12- or 60-month concentration of or the mean 12-month concentration of prior to the baseline visit for each subject (. Analyses were restricted to exposures within the prior 12 months because data were only available since 2015). Because the development of depression occurred at an unknown time point between two exam visits, we used flexible parametric proportional hazards models using natural cubic splines in log-time to account for this type of interval censoring (Royston and Parmar 2002).
We adjusted for a priori factors that could potentially confound the association between air pollution and developing depression. We used a base model adjusted for age, sex, and study center (Seoul or Suwon) and a fully adjusted model further adjusted for smoking status, alcohol consumption, physical activity, and body mass index. We investigated potential effect modification by age group ( of age), sex, education (university and less than university, including no education, elementary school, middle school, high school, and technical college), BMI (underweight, normal, overweight, obese, and unknown), alcohol intake (none, moderate drinking, excessive drinking, and unknown), and physical activity (none, , , and unknown). For each potential effect modifier, we evaluated effect modification by likelihood ratio tests comparing models that included an interaction (product) term between air pollution exposure and the effect modifier versus models without the interaction term. Stratum-specific HRs were obtained from the same interaction model by using the appropriate coefficients and variance-covariance matrix.
To quantify the effect of unmeasured potential confounding factors, we report the E-value as a representation of the minimum strength of association that an unmeasured confounder would need to have with the exposure and outcome to nullify an observed exposure–outcome association. Increasing E-values indicate that a higher degree of unmeasured confounding would be needed to reduce the observed association to the null (VanderWeele and Ding 2017).
To evaluate nonlinear dose–response relationships between and exposure and incident depression, we modeled and air pollution exposure variables using restricted cubic splines with knots at the fifth, 35th, 65th, and 95th percentiles of the distribution of PM air pollution concentrations. Statistical analyses were conducted using STATA (version 15.0; Stata Corporation) and R (version 3.4.1; R Development Core Team).
Results
The mean age of the cohort at baseline was 39.4 y [standard deviation (SD) 6.8], and 60.1% of the subjects were men (Table 1). The mean (SD) concentrations of during the 12- and 60-month periods prior to the baseline visit were 50.6 (4.5) and , respectively, and the mean 12-month concentration of was . The prevalence of overweight/obesity was 51.3%, and 22.0% of study subjects were current smokers. At the baseline visit, the mean (SD) CES-D score was 5.1 (4.2), and 9.7% of study subjects developed CES-D scores during follow-up. Seven percent of the study sample had missing data for CES-D scores. Compared with subjects missing CES-D data, those included in the study were, on mean, younger, more likely to be male, and less likely to have missing data for smoking, alcohol, physical activity, and educational level. There was no material difference in PM air pollution levels (see Table S1).
Table 1.
Baseline characteristics of Kangbuk Samsung Health Study (KSHS) cohort subjects, overall and according to the incidence of depression (CES-D score ) during follow-up.
| Characteristic | Overall | CESD Score | p-Valuea | |
|---|---|---|---|---|
| n | 123,045 | 111,141 | 11,904 | |
| Age (y) | 39.4 (6.8) | 39.3 (6.8) | 39.5 (6.5) | 0.11 |
| Male sex | 73,930 (60.1) | 67,594 (60.8) | 6,336 (53.2) | |
| 12-month mean () | 24.3 (1.3) | 24.2 (1.3) | 24.2 (1.2) | |
| 12-month mean () | 50.6 (4.5) | 50.6 (4.4) | 51.4 (4.5) | |
| 60-month mean () | 55.2 (4.0) | 55.2 (4.0) | 55.8 (4.0) | |
| BMI () | ||||
| 6,203 (5.0) | 5,504 (5.0) | 699 (5.9) | ||
| 53,368 (43.4) | 48,066 (43.2) | 5,302 (44.5) | ||
| 28,317 (23.0) | 25,737 (23.2) | 2,580 (21.7) | ||
| 34,870 (28.3) | 31,587 (28.4) | 3,283 (27.6) | ||
| Unknown | 287 (0.2) | 247 (0.2) | 40 (0.3) | |
| Smoking status | ||||
| Never smoker | 54,723 (44.5) | 49,424 (44.5) | 5,299 (44.5) | |
| Former smoker | 27,151 (22.1) | 24,995 (22.5) | 2,156 (18.1) | |
| Current smoker | 27,095 (22.0) | 24,315 (21.9) | 2,780 (23.4) | |
| Unknown | 14,076 (11.4) | 12,407 (11.2) | 1,669 (14.0) | |
| Education | ||||
| No education | 28 (0.0) | 23 (0.0) | 5 (0.0) | |
| Elementary school | 216 (0.2) | 199 (0.2) | 17 (0.1) | |
| Middle school | 527 (0.4) | 452 (0.4) | 75 (0.6) | |
| High school | 14,972 (12.2) | 13,280 (11.9) | 1,692 (14.2) | |
| Technical college | 14,609 (11.9) | 13,058 (11.7) | 1,551 (13.0) | |
| University | 88,181 (71.7) | 80,131 (72.1) | 8,050 (67.6) | |
| Unknown | 4,512 (3.7) | 3,998 (3.6) | 514 (4.3) | |
| Alcohol intakeb | ||||
| None | 16,552 (13.5) | 15,050 (13.5) | 1,502 (12.6) | |
| Moderate | 81,672 (66.4) | 73,922 (66.5) | 7,750 (65.1) | |
| High | 17,159 (13.9) | 15,402 (13.9) | 1,757 (14.8) | |
| Unknown | 7,662 (6.2) | 6,767 (6.1) | 895 (7.5) | |
| Daily physical activity | 0.002 | |||
| None | 72,092 (58.6) | 64,961 (58.4) | 7,131 (59.9) | |
| 40,048 (32.5) | 36,359 (32.7) | 3,689 (31.0) | ||
| 7,554 (6.1) | 6,804 (6.1) | 750 (6.3) | ||
| Unknown | 3,351 (2.7) | 3,017 (2.7) | 334 (2.8) | |
Note: Numbers in the table are mean (SD) or n (%). ANOVA, analysis of variance; CES-D, Center for Epidemiological Studies Depression scale.
p-Value for differences of means of proportions comparing CES-D score and CES-D score group, calculated using one-way ANOVA for continuous variables and chi-square test for categorical variables. The data were complete for continuous variables.
Frequency of alcohol consumption was categorized as none, moderate drinking (men: , women: ), excessive drinking (men: , women ), and unknown.
PM Air Pollution and the Risk of Developing Depression
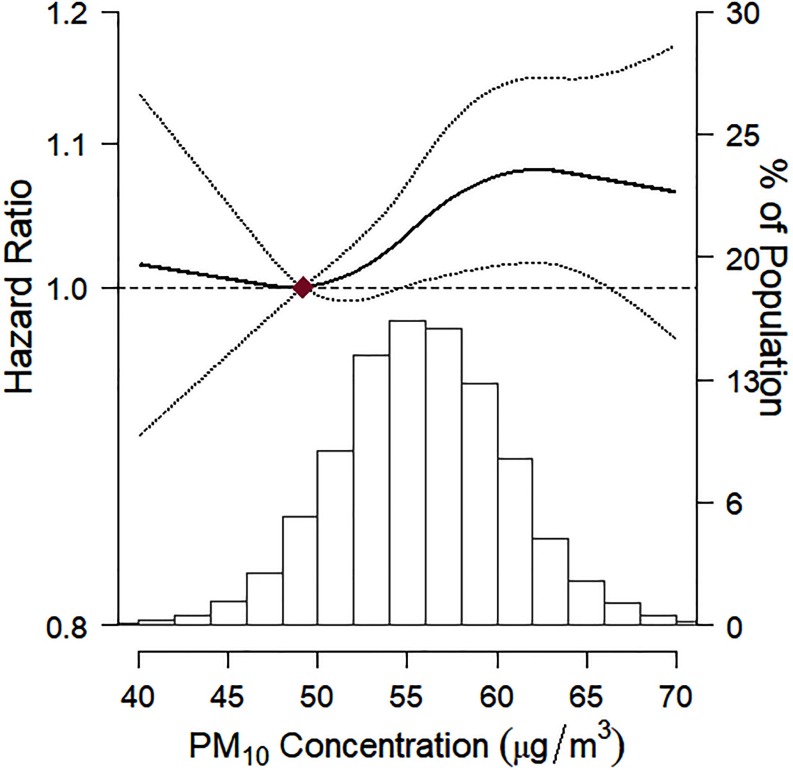
Among 123,045 subjects with baseline CES-D score and at least one follow-up visit, 11,904 subjects developed new-onset depression over follow-up (mean follow-up 2.5 y; 310,075 person-years of follow-up). In fully adjusted models, the HRs (95% CIs) for the development of depression associated with a increase in 12- and 60-month exposure were 1.11 (95% CI: 1.06, 1.16) and 1.06 (95% CI: 1.01, 1.11), respectively (Table 2). The HRs comparing the top to the bottom quintile of 12- and 60-month exposure were 1.14 (95% CI: 1.07, 1.22) and 1.08 (95% CI: 1.01, 1.15), respectively. The E-value for the observed association estimates of exposure to 12-month exposure and incident depression was 1.46 (1.37 for the lower confidence interval). In spline regression analyses, the risk of developing depression increased with increasing 60-month mean exposure throughout most of the distribution of concentrations (Figure 2).
Table 2.
Hazard ratios (95% confidence intervals) for developing depression for a increase in particulate matter air pollution in the Kangbuk Samsung Health Study (KSHS).
| Developing depression assessment method | Exposure | Cases/person-yearsa | Adjustment | 12-month per increase | 60-month per increase | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Developing depression () | 11,904/310,075 | Model 1b | 1.13 | 1.08, 1.18 | 1.08 | 1.03, 1.14 | |
| Model 2c | 1.11 | 1.06, 1.16 | 1.06 | 1.01, 1.11 | |||
| 2,647/56,719 | Model 1 | 1.01 | 0.83, 1.22 | — | — | ||
| Model 2 | 1.01 | 0.83, 1.22 | — | — | |||
| Developing depression (doctor’s diagnosis or use of medications) | 1,126/312,334 | Model 1 | 1.21 | 1.02, 1.45 | 1.2 | 1.00, 1.44 | |
| Model 2 | 1.21 | 1.01, 1.45 | 1.2 | 1.00, 1.43 | |||
| 402/56,646 | Model 1 | 0.93 | 0.63, 1.38 | — | — | ||
| Model 2 | 0.96 | 0.64, 1.43 | — | — | |||
Note: Estimates represent HRs (95% CIs) for as a dichotomous outcome. —, data not available; CES-D, Center for Epidemiological Studies Depression scale; CI, confidence interval; HR, hazard ratio; , particulate matter with an aerodynamic diameter of ; , particulate matter with an aerodynamic diameter of .
Person-years were the total numbers for all subjects and account for missing covariate data. The person-years and number of cases were different between and models because the follow-up for model started on 1 January 2011 and the follow-up for model started on 1 January 2015.
Model 1 was adjusted for age, sex, study center, and year of visit.
Model 2 was adjusted as for Model 1 and additionally adjusted for educational level, smoking status, body mass index, alcohol consumption, and physical activity.
Figure 2.
Hazard ratio (95% confidence interval) for incident depression by level of exposure to 60-month concentrations. Incident depression was defined as the development of a CES-D score over follow-up. The dose–response curve was calculated using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of the distribution of 60-month concentrations. The solid line represents the hazard ratios, and the dotted lines represent the confidence interval limits. The reference exposure level was set at the 10th percentile of the distribution of 60-month concentrations (). Hazard ratios were adjusted for age, sex, study center, year of visit, educational level, smoking status, body mass index, alcohol consumption, and physical activity. The histogram illustrates the distribution of 60-month concentrations. , particulate matter with an aerodynamic diameter of .
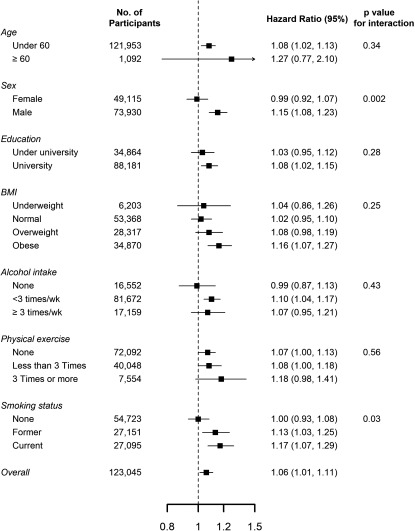
When we defined incident depression based on self-reports of doctor’s diagnosis or use of antidepressant medications, 1,126 subjects developed new-onset depression over follow-up (mean follow-up 2.7 y; 312,334 person-years of follow-up), the HRs were similar to those in the main analysis (Table 2). In subgroup analyses, the association between exposure to and the developing depression was stronger in men compared with women and in former and current smokers compared with nonsmokers (Figure 3).
Figure 3.
Hazard ratios (95% confidence intervals) for incident depression () associated with a increase in 60-month concentrations, by baseline subject characteristics. Hazard ratios were adjusted for age, sex, study center, year of visit, educational level, smoking status, body mass index, alcohol consumption, and physical activity. Education was categorized as university () and less than university (, including no education, elementary school, middle school, high school, and technical college). p-Values were derived from likelihood ratio tests comparing models that included an interaction (product) term between air pollution exposure and the effect modifier vs. models without the interaction term. Results for missing categories are not shown because some of these categories were very small.
data were only available beginning in 2015. The analyses for were thus based on 55,142 subjects with a CES-D score of after 1 January 2015 and at least one follow-up visit. Among them, 2,647 subjects developed new onset of depression over follow-up (mean follow-up 1.0 y; 56,719 person-years of follow-up; Table 2). In fully adjusted models, the HR for the development of depression associated with a increase in 12-month concentrations was 1.01 (95% CI: 0.83, 1.22). The hazard ratio comparing the top quintile to the bottom quintile of 12-month mean concentrations was 1.04 (95% CI: 0.91, 1.20). In spline regression analysis, there appeared to be a bell-shaped association between 12-month mean and risk of depression, although the p-value for nonlinear terms was not statistically significant (see Figure S1).
As a sensitivity analysis, when we evaluated the association between exposure and development of depression restricted to the same time frame as the analyses (see Table S2), the association was virtually null. However, when restricting the analysis to same group of subjects as the analyses but with follow-up starting in 2011, the association of with the incidence of depression persisted.
Discussion
In this large, prospective study of middle-aged Korean men and women, long-term exposure to was associated with an increased risk of developing depression over follow-up. The results were consistent when we defined the development of depression using CES-D data and when we defined the cases used self-reports of doctor’s diagnoses or use of antidepressant medications. These findings support an association between air pollution and an increased risk of depression.
Prior studies of the association between air pollution and depression have been inconsistent, with differences based partly on the method used to identify new cases of depression. In a general population study in Korea, exposure was associated with an increased risk of depressive disorder defined as a doctor’s diagnosis or use of antidepressant medications in subjects with underlying chronic diseases (Kim et al. 2016). In the Nurses’ Health Study, however, the association between air pollution and onset of depression was only evident when depression was identified as antidepressant medication use and not when incident cases were identified as a doctor’s diagnosis or as the combination of a doctor’s diagnosis and medication use (Kioumourtzoglou et al. 2017). Two cross-sectional studies that defined depression by self-reported history also identified an association between long-term exposure to air pollution and depression (Lin et al. 2017; Vert et al. 2017). In contrast, the Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston (MOBILIZE) Boston cohort (), which identified incident cases of depression using CES-D scores, and the FINRISK study (), which identified prevalent cases of depression using CES-D scores, found no association with air pollution (Wang et al. 2014; Zijlema et al. 2016). Finally, a cohort study () of elderly subjects identified a positive association between exposure and development of moderate-to-severe depressive symptoms based on CES-D, but the use of the short version of the CES-D scale (11-items) form, the high prevalence of comorbidities, and the advanced age of the study subjects limited the generalizability of these findings (Pun et al. 2017). Our study is the only large cohort study that has simultaneous information on CES-D scores, self-reports of doctor’s diagnosis of depression, and use of antidepressant medications. Our analysis indicated that air pollution is associated with incident depression irrespective of the method used to assess the outcome, adding robustness to these findings.
Several mechanisms may underlie the association between air pollution and depression. PM could affect the central nervous system through multiple mechanisms, including disruption of the blood–brain barrier, endothelial dysfunction, DNA damage, and the formation of free radicals and oxidative stress (Brun et al. 2012; Calderón-Garcidueñas et al. 2015; Campbell 2004; Fonken et al. 2011; Guo et al. 2012; Levesque et al. 2011; Wu et al. 2011). A growing body of toxicological studies suggests that chronic exposure to PM and ozone are related to cognitive and mental disorders in experimental animals, including depression-like behavior (Jones and Thomsen 2013; Mokoena et al. 2015; Oberdörster et al. 2004). One study in mice, for instance, reported that prolonged exposure to PM air pollution may result in neuroinflammation, altered morphological characteristics in hippocampal neurons, changes in affective behaviors, and cognitive impairment (Fonken et al. 2011). In humans, it has been suggested that PM could enter the systemic circulation, reach the brain, and result in oxidative stress and inflammation in the central nervous system (Calderón-Garcidueñas et al. 2009; Valavanidis et al. 2008), and there is evidence that neurochemical changes related to inflammatory factors may play an important role in the development of depression (Anisman and Hayley 2012; Raison and Miller 2011). Additional research, however, is needed to better establish the precise mechanisms that underlie the association between air pollution and depression.
We did not observe an association between exposure and development of depression in this study, but this could be due to the short follow-up for analysis, to the smaller sample size, to chance, or to the presence of unmeasured effect modifiers that changed over time. Additional studies with longer follow-up periods are required to evaluate this association.
In the stratified analyses, we estimated stronger associations of air pollution in men compared with women. These differences may be the result of biological differences related to hormonal status or body size, to differences in co-exposures such as work-related exposures or smoking, or to differences in outdoor exposure patterns (Abbey et al. 1998; Clougherty 2010). We also found a stronger association between exposure and incident depression in subjects who reported current smoking compared with those who reported former smoking and no smoking, suggesting a synergistic association between air pollution and smoking.
The strengths of this study included a prospective design, a large sample size, and the availability of information on a wide range of potential confounders. Most of the subjects appeared to be healthy workers with few comorbid conditions and a low prevalence of medication use. In addition, we had two measurements of incident depression available, including self-reports of diagnosis of depression and/or use of antidepressant medications and CES-D scores. The consistency of the findings across two measures adds strength to our findings of an adverse effect of PM air pollution on depressive disorders.
Our findings should be interpreted with consideration of some limitations. Although the land-use regression model is a well-validated method for assessing the chronic adverse effects of air pollution in epidemiological studies, PM concentrations were assigned in this study based on the postal code centroid for the residential address for each subject at the beginning of follow-up. Although the cross-validated of the models were high, our prediction models for air pollution could still result in measurement error. In addition, we were not able to adjust for neighborhood-level socioeconomic status in our analyses, which may be a confounder of the association between ambient air pollution and health outcomes (Havard et al. 2009). As a result, our results could be biased by residual confounding. As an additional limitation, we did not have data on mobility, workplace exposure, level of urbanicity, traffic noise, or change in residential address over follow-up. These factors could potentially contribute to confounding or to measurement error that could bias the estimated association between air pollution exposure and the developing depression (Hart et al. 2015a; Kioumourtzoglou et al. 2014; Zeger et al. 2000).
In South Korea, the main sources of pollution are motor vehicles, soil dust, and combustion/industry, whereas the main sources of are motor vehicles and soil dust (Ryou et al. 2018). Because most of the subjects resided and worked in the urban area of Seoul and Suwon, local motor traffic is likely the primary source of air pollution for the subjects in this study. Given the relatively high air pollution concentration in Asia, investigation of the relation between air pollution and developing depression in cohort studies from other geographical areas is needed. In addition, this study group primarily comprised relatively healthy and highly educated young and middle-aged Korean workers who attended health screening exams, and, thus, our results may not be generalizable to other populations or to other settings.
Conclusions
In this large cohort study, we found a positive association between long-term exposure to outdoor PM air pollution and developing depression. Our results were consistent across two methods to define incident cases of depression, suggesting that long-term air pollution exposure may be linked not only to cases of depression that seek medical care but also to depressive episodes outside of the health care system. Our findings add further evidence to recent data suggesting that air pollution is responsible for a variety of cognitive and neuropsychiatric conditions and may be a major determinant of mental health in the general population.
Supplementary Material
Acknowledgments
We thank T.S. Choi (Kangbuk Samsung Hospital, Information System, Seoul, Korea) for his help with technical support in gathering data. This study received no external funding.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4094).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abbey DE, Burchette RJ, Knutsen SF, McDonnell WF, Lebowitz MD, Enright PL. 1998. Long-term particulate and other air pollutants and lung function in nonsmokers. Am J Respir Crit Care Med 158(1):289–298, PMID: 9655742, 10.1164/ajrccm.158.1.9710101. [DOI] [PubMed] [Google Scholar]
- Ailshire JA, Crimmins EM. 2014. Fine particulate matter air pollution and cognitive function among older US adults. Am J Epidemiol 180(4):359–366, PMID: 24966214, 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Hayley S. 2012. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal 5(244):pe45, PMID: 23033537, 10.1126/scisignal.2003579. [DOI] [PubMed] [Google Scholar]
- Bell RA, Franks P, Duberstein PR, Epstein RM, Feldman MD, Fernandez y Garcia E, et al. . 2011. Suffering in silence: reasons for not disclosing depression in primary care. Ann Fam Med 9(5):439–446, PMID: 21911763, 10.1370/afm.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Paterniti DA, Azari R, Duberstein PR, Epstein RM, Rochlen AB, et al. . 2010. Encouraging patients with depressive symptoms to seek care: a mixed methods approach to message development. Patient Educ Couns 78(2):198–205, PMID: 19674862, 10.1016/j.pec.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun E, Carrière M, Mabondzo A. 2012. In vitro evidence of dysregulation of blood–brain barrier function after acute and repeated/long-term exposure to TiO2 nanoparticles. Biomaterials 33(3):886–896, PMID: 22027597, 10.1016/j.biomaterials.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Calderón-Garcidueñas A, Torres-Jardón R, Avila-Ramírez J, Kulesza RJ, Angiulli AD. 2015. Air pollution and your brain: what do you need to know right now. Primary Health Care Res Dev 16(4):329–345, PMID: 25256239, 10.1017/S146342361400036X. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Macías-Parra M, Hoffmann HJ, Valencia-Salazar G, Henríquez-Roldán C, Osnaya N, et al. . 2009. Immunotoxicity and environment: immunodysregulation and systemic inflammation in children. Toxicol Pathol 37(2):161–169, PMID: 19171930, 10.1177/0192623308329340. [DOI] [PubMed] [Google Scholar]
- Campbell A. 2004. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci 1035:117–132, PMID: 15681804, 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Chun MY. 2012. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med 33(3):144–151, PMID: 22787536, 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE. 2010. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect 118(2):167–176, PMID: 20123621, 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. . 2017. Air pollution and mortality in the medicare population. N Engl J Med 376(26):2513–2522, PMID: 28657878, 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, et al. . 2011. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry 16(10):987–995, PMID: 21727897, 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhu N, Guo Z, Li GK, Chen C, Sang N, et al. . 2012. Particulate matter (PM10) exposure induces endothelial dysfunction and inflammation in rat brain. J Hazard Mater 213–214:28–37, PMID: 22365138, 10.1016/j.jhazmat.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Hart JE, Liao X, Hong B, Puett RC, Yanosky JD, Suh H, et al. . 2015a. The association of long-term exposure to PM2.5 on all-cause mortality in the Nurses’ Health Study and the impact of measurement-error correction. Environ Health 14:38, PMID: 25926123, 10.1186/s12940-015-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. 2015b. Effect modification of long-term air pollution exposures and the risk of incident cardiovascular disease in US women. J Am Heart Assoc 4(12):e002301, PMID: 26607712, 10.1161/JAHA.115.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havard S, Deguen S, Zmirou-Navier D, Schillinger C, Bard D. 2009. Traffic-related air pollution and socioeconomic status: a spatial autocorrelation study to assess environmental equity on a small-area scale. Epidemiology 20(2):223–230, PMID: 19142163, 10.1097/EDE.0b013e31819464e1. [DOI] [PubMed] [Google Scholar]
- James P, Hart JE, Banay RF, Laden F, Signorello LB. 2017. Built environment and depression in low-income African Americans and whites. Am J Prev Med 52(1):74–84, PMID: 27720338, 10.1016/j.amepre.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SW, Lim SW, Shin DW, Ryu S, Chang Y, Kim SY, Oh KS1, Shin YC, Kim YH. 2019. Metabolic syndrome and incident depressive symptoms in young and middle-aged adults: A cohort study. J Affect Disord 1; 246:643–651, PMID: 30611062, 10.1016/j.jad.2018.12.073. [DOI] [PubMed] [Google Scholar]
- Jones KA, Thomsen C. 2013. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci 53:52–62, PMID: 23064447, 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kim JS, Zhang Y, Chang Y, Ryu S, Guallar E, Shin Y-C, et al. . 2018. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab 103(5):1827–1833, PMID: 29408972, 10.1210/jc.2017-01247. [DOI] [PubMed] [Google Scholar]
- Kim K-N, Lim Y-H, Bae HJ, Kim M, Jung K, Hong Y-C. 2016. Long-term fine particulate matter exposure and major depressive disorder in a community-based urban cohort. Environ Health Perspect 124(10):1547–1553, PMID: 27129131, 10.1289/EHP192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-D, Hong S-C, Lee C-I, Kwak Y-S, Shin T-K, Jang Y-H, et al. . 2007. Prevalence of depression and correlates of depressive symptoms for residents in the urban part of Jeju Island, Korea. Int J Soc Psychiatry 53(2):123–134, PMID: 17472086, 10.1177/0020764006075022. [DOI] [PubMed] [Google Scholar]
- Kioumourtzoglou M-A, Power MC, Hart JE, Okereke OI, Coull BA, Laden F, et al. . 2017. The association between air pollution and onset of depression among middle-aged and older women. Am J Epidemiol 185(9):801–809, PMID: 28369173, 10.1093/aje/kww163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou M-A, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. . 2014. Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environ Health 13(1):2, PMID: 24410940, 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Surace MJ, McDonald J, Block ML. 2011. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation 8:105, PMID: 21864400, 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Guo Y, Kowal P, Airhihenbuwa CO, Di Q, Zheng Y, et al. . 2017. Exposure to air pollution and tobacco smoking and their combined effects on depression in six low-and middle-income countries. Br J Psychiatry 211(3):157–162, PMID: 28798061, 10.1192/bjp.bp.117.202325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena ML, Harvey BH, Viljoen F, Ellis SM, Brink CB. 2015. Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology (Berl) 232(16):2921–2938, PMID: 25877744, 10.1007/s00213-015-3928-8. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. . 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16(6–7):437–445, PMID: 15204759, 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. 2014. Depression in the U.S. household population, 2009–2012. NCHS Data Brief 172:1–8, PMID: 25470183. [PubMed] [Google Scholar]
- Pun VC, Manjourides J, Suh H. 2017. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the NSHAP study. Environ Health Perspect 125(3):342–348, PMID: 27517877, 10.1289/EHP494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401, 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raison CL, Miller AH. 2011. Is depression an inflammatory disorder? Curr Psychiatry Rep 13(6):467–475, PMID: 21927805, 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Parmar MK. 2002. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 21(15):2175–2197, PMID: 12210632, 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- Ryou HG, Heo J, Kim SY. 2018. Source apportionment of PM10 and PM2.5 air pollution, and possible impacts of study characteristics in South Korea. Environ Pollut 240:963–972, PMID: 30223380, 10.1016/j.envpol.2018.03.066. [DOI] [PubMed] [Google Scholar]
- Simon GE. 2003. Social and economic burden of mood disorders. Biol Psychiatry 54(3):208–215, PMID: 12893097, 10.1016/S0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M, Kousha T, Kingsbury M, Colman I. 2016. Air pollution and emergency department visits for depression: a multicity case-crossover study. Environ Health Insights 10:155–161, PMID: 27597809, 10.4137/EHI.S40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. 2008. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26(4):339–362, PMID: 19034792, 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Ding P. 2017. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 167(4):268–274, PMID: 28693043, 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- Vert C, Sánchez-Benavides G, Martínez D, Gotsens X, Gramunt N, Cirach M, et al. . 2017. Effect of long-term exposure to air pollution on anxiety and depression in adults: a cross-sectional study. Int J Hyg Environ Health 220(6):1074–1080, PMID: 28705430, 10.1016/j.ijheh.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Koutrakis P, Gryparis A, Schwartz JD, Coull BA, et al. . 2014. Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect 122(6):553–558, PMID: 24610154, 10.1289/ehp.1205909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. Depression. http://www.Who.Int/mediacentre/factsheets/fs369/en/ [accessed 16 February 2018].
- Wong DFK, Xuesong H, Poon A, Lam AYK. 2012. Depression literacy among Chinese in Shanghai, China: a comparison with Chinese-speaking Australians in Melbourne and Chinese in Hong Kong. Soc Psychiatry Psychiatr Epidemiol 47(8):1235–1242, PMID: 21901401, 10.1007/s00127-011-0430-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang C, Sun J, Xue Y. 2011. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano 5(6):4476–4489, PMID: 21526751, 10.1021/nn103530b. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. . 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13:63, PMID: 25097007, 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. . 2000. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 108(5):419–426, PMID: 10811568, 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Laden F, Forman JP, Hart JE. 2016. Long-term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses’ Health Study. Environ Health Perspect 124(9):1414–1420, PMID: 27177127, 10.1289/EHP163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Hart JE, Laden F, Zhao C, Li T, et al. . 2018. National scale spatiotemporal land-use regression model for PM2.5, PM10 and NO2 concentration in China. Atmos Environ 192:48–54, 10.1016/j.atmosenv.2018.08.046. [DOI] [Google Scholar]
- Zijlema W, Wolf K, Emeny R, Ladwig K, Peters A, Kongsgård H, et al. . 2016. The association of air pollution and depressed mood in 70,928 individuals from four European cohorts. Int J Hyg Environ Health 219(2):212–219, PMID: 26682644, 10.1016/j.ijheh.2015.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.