Abstract
Background:
Identifying factors that impair bone accrual during childhood is a critical step toward osteoporosis prevention. Exposure to per- and polyfluoroalkyl substances (PFASs) has been associated with lower bone mineral density, but data are limited, particularly in children.
Methods:
We studied 576 children in Project Viva, a Boston-area cohort of mother/child pairs recruited prenatally from 1999 to 2002. We quantified plasma concentrations of several PFASs and measured areal bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) in midchildhood. We used linear regression to examine associations between plasma concentrations of individual PFASs and aBMD z-score. We used weighted quantile sum (WQS) regression to examine the association of the PFAS mixture with aBMD z-score. All models were adjusted for maternal age, education, annual household income, census tract median household income, and child age, sex, race/ethnicity, dairy intake, physical activity, and year of blood draw.
Results:
Children were [] of age. The highest PFAS plasma concentrations were of perfluorooctanesulfonic acid (PFOS) {median [interquartile range (IQR)]: 6.4 (5.6) ng/mL} and perfluorooctanoic acid (PFOA) [median (IQR): 4.4 (3.2) ng/mL]. Using linear regression, children with higher plasma concentrations of PFOA, PFOS, and perfluorodecanoate (PFDA) had lower aBMD z-scores [e.g., : ; 95% confidence interval (CI): , per doubling of PFOA]. The PFAS mixture was negatively associated with aBMD z-score (: ; 95% CI: , per IQR increment of the mixture index).
Conclusions:
PFAS exposure may impair bone accrual in childhood and peak bone mass, an important determinant of lifelong skeletal health. https://doi.org/10.1289/EHP4918
Introduction
Osteoporosis affects over 200 million adults worldwide, and associated fractures are costly, with high morbidity and mortality and limited treatment options (Reginster and Burlet 2006). Because bone accrues during childhood and adolescence and is maximized by early adulthood (Gordon et al. 2017), identifying and remediating factors that lead to low bone accrual in childhood is a critical component of osteoporosis prevention efforts. However, our understanding of the role of environmental factors on childhood bone accrual is limited.
Per- and polyfluoroalkyl substances (PFASs), ubiquitous environmental contaminants added to food packaging, clothing, furniture, and carpets to make the products nonstick and stain repellant (Lindstrom et al. 2011), are universally detectable in the general U.S. population, including children (Calafat et al. 2007; Ye et al. 2018).
PFASs may influence areal bone mineral density (aBMD) through several mechanisms. PFASs activate the nuclear peroxisome proliferator–activated receptor gamma (), which suppresses the osteoblast lineage of mesenchymal stem cells (Yamamoto et al. 2015). PFASs may also reduce BMD by acting as androgen receptor antagonists (Clarke and Khosla 2009; Kjeldsen and Bonefeld-Jorgensen 2013), inhibiting steroidogenic enzyme activity on the androgen secretion pathway (Zhao et al. 2010) or by directly intercalating into bone (Bogdanska et al. 2011; Koskela et al. 2017; Pérez et al. 2013). Consistent with these mechanistic data, two epidemiologic studies in U.S. adults reported higher PFAS serum concentrations to be associated with lower aBMD (Khalil et al. 2016; Lin et al. 2014), and in a pilot cross-sectional study of 48 obese children, higher PFAS plasma concentrations were associated with lower aBMD measured by calcaneal quantitative ultrasound (Khalil et al. 2018).
The extent to which different PFASs may affect aBMD in children is not well characterized. Based on limited existing data suggesting a contemporaneous inverse association between PFAS exposure and aBMD, we set out to test the hypothesis that higher plasma PFAS concentrations would be cross-sectionally associated with lower aBMD z-score at midchildhood in a large Boston-area cohort.
Methods
Study Population and Design
Between 1999 and 2002, we recruited pregnant women to Project Viva, a longitudinal cohort study of prenatal exposures and child health, during their first prenatal visit at Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts (Oken et al. 2015). Of 2,128 mother–infant pairs, 1,116 (54%) children had a follow-up research visit in midchildhood (age range, 6–10 y; median, 7.7 y). At the midchildhood visit, 653 (59%) of these children had PFAS concentrations measured in plasma, and 576 children from this population had aBMD measured by dual-energy X-ray absorptiometry (DXA) and thus were included in the present analysis. Among participants who attended the midchildhood visit, participants who were included in the statistical analysis were less likely to be white compared with excluded participants (57% vs. 70%). Although included participants’ mothers were less likely to have graduated from college (64% vs. 72%), they were more likely to belong to households with high income (total annual income ) compared with participants who were excluded (80% vs. 68%) (see Table S1).
The institutional review boards of participating institutions approved this analysis. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research. All mothers provided written informed consent for their child’s participation.
Exposure and Outcome Measurements
We measured several PFASs in plasma collected in midchildhood as previously described (Sagiv et al. 2015). Staff at the Division of Laboratory Sciences at the CDC quantified PFASs using online solid-phase extraction with isotope dilution high-performance liquid chromatography–mass spectrometry. The limit of detection (LOD) was for all PFASs; we replaced values below the LOD with the . We summed the concentrations of the isomers of perfluorooctanoic acid (PFOA) [i.e., n-PFOA and the sum of perfluoromethylheptanoates and perfluorodimethylhexanoates (Sb-PFOA)] to obtain total PFOA. We summed the concentrations of the isomers of perfluorooctanesulfonic acid (PFOS) [i.e., n-PFOS, sum of perfluoromethylheptane sulfonates (Sm-PFOS), and sum of perfluorodimethylhexane sulfonates (Sm2-PFOS)] to obtain total PFOS. We decided a priori to only consider PFASs with detectable concentrations in of samples for the current analysis. The PFASs that met this threshold were n-PFOA, n-PFOS, Sm-PFOS, total PFOA, total PFOS, perfluorodecanoate (PFDA, previously abbreviated as PFDeA), perfluorohexane sulfonate (PFHxS), 2-(N-methyl-perfluorooctane sulfonamide) acetate (MeFOSAA, previously abbreviated as MePFOSA-AcOH), and perfluorononanoate (PFNA).
We measured total-body (excluding the skull) aBMD and bone mineral content (BMC) in midchildhood via a total-body DXA scan (Discovery A model; Hologic Inc.). In order to avoid variation in aBMD measures between DXA instruments (Brownbill and Ilich 2005), we used the same DXA scanner for all participants and calibrated it daily with a standard phantom from the manufacturer to assess for machine drift. We excluded the skull in the measurements because the skull comprises a large proportion of the skeleton in growing children and is not responsive to physical activity or other environmental influences (Crabtree et al. 2014). Total body less head measurements are also recommended for clinical pediatric skeletal assessments (Gordon et al. 2014). Intrarater reliability on a subset of measurements was high . We analyzed data with Hologic software (version 12.6) using the pediatric setting and used U.S. national reference data to derive age-, sex-, race-, and height-adjusted aBMD and BMC z-scores (Zemel et al. 2011).
Covariates
We administered questionnaires to collect data on maternal education, parity, smoking habits during pregnancy and child secondhand smoke exposure, breastfeeding duration, annual household income, and race/ethnicity. At the midchildhood visit, we used a PrimeScreen questionnaire to collect information on the child’s soda and dairy (milk, yogurt, cheese, butter, and ice cream) intake in servings per day over the past month (Rifas-Shiman et al. 2001). In a subset of children (), we measured serum 25-hydroxyvitamin D [25(OH)D] using isotope dilution liquid chromatography–tandem mass spectrometry (Baker et al. 2011; Singh et al. 2006). Based on home address recorded at the midchildhood visit, we obtained median annual household income for the child’s residential census tract from U.S. Census data (U.S. Census Bureau 2000).
Statistical Analyses
We first ran single-PFAS linear regression models to examine independent associations between each PFAS and aBMD z-score. Next, we ran a multi-PFAS linear regression model to examine associations between each PFAS and aBMD z-score, independent of the other PFASs. Finally, we examined the association between the PFAS mixture and aBMD z-score via weighted quantile sum (WQS) regression. The distributions of all plasma PFAS concentrations were right skewed. To evaluate nonlinearity, we fit penalized spline generalized additive models of the associations of individual PFASs with aBMD z-scores. Associations were linear when PFAS plasma concentrations were transformed. We therefore used -transformed PFAS plasma concentrations in our linear regression analyses and in developing a composite weighted index in the WQS analysis.
In all models, we accounted for potential positive or negative confounders of the association between PFAS plasma concentration (Sagiv et al. 2015) and aBMD z-score (Rubin et al. 1993). We evaluated linearity of the relationship between each continuous covariate and the outcome using penalized spline generalized additive models, and we categorized covariates that were nonlinearly associated with aBMD z-score. The covariates that we included in our final models were maternal age at enrollment (years), maternal education (with or without college degree), individual household income (, , ), census tract median household income (, , , ), child age (years), sex (dichotomous), race/ethnicity (white, black, Asian, Hispanic, other), dairy intake (servings per week), physical activity (hours per week), and year of blood draw for PFAS measurement (2007–2010). We included census tract median household income in addition to individual household income data because we have previously shown census tract median household income to be more strongly associated with PFAS concentrations (Harris et al. 2017). Collinearity between the income variables did not impact the results, based on variance inflation factors in the final models ( for each category of census tract median household income, and for each category of individual household income).
We considered but did not include the following variables (see Table S2 for descriptive data): breastfeeding duration, soda intake, secondhand smoke exposure, and 25(OH)D plasma concentration. Figure S1 demonstrates that these variables did not confound the PFAS–aBMD association (i.e., estimate for the primary exposure changed by ). We did not consider pubertal status or body mass index (BMI) z-score/adiposity, as these variables may be on the causal pathway between PFAS exposure and aBMD z-score (Gilsanz et al. 2011; Lopez-Espinosa et al. 2011; Domazet et al. 2016), and inclusion in the analysis could introduce collider bias (Cole et al. 2010). We substituted maternal race/ethnicity for the 10% of children in the cohort who were missing data on race/ethnicity. We performed complete case analyses. Among participants with available exposure/outcome data, 92.2% had information on all covariates and thus were included in our models.
In our single- and multi-PFAS linear regression models, we examined associations between the following individual PFASs and aBMD z-score: total PFOA, total PFOS, PFDA, PFHxS, MeFOSAA, and PFNA. Next, we used WQS to estimate the association between the PFAS mixture and aBMD z-score. WQS assigns each PFAS within the mixture a weight that reflects the strength of that PFAS–outcome association. However, the weight of each PFAS is also affected by the collinearity between that PFAS and other PFASs in the mixture, such that a given PFAS will have a lower weight if it is highly correlated with another PFAS in the mixture. In this case, the sum of the two weights reflect the contribution of the correlated components to the overall mixture effect. (Carrico et al. 2015).Our WQS model takes the form , where is the decile corresponding to the jth exposure for the ith person, is a weight for the jth exposure estimated by 500 bootstraps, and represents the overall effect of the mixture. We constrained weights to sum to 1.0 so that all mixture components could be incorporated into a single effect estimate, we constrained to be negative based on findings in individual PFAS models, and we scaled by interquartile range (IQR) of the WQS index to improve interpretability.
We assessed the robustness of our findings through multiple sensitivity analyses of the single-PFAS linear regression models. To control for prenatal PFAS exposure, we adjusted final models for -transformed maternal PFAS concentration in early pregnancy, measured at a median of 10 wk gestation (except for the model of PFDA, as PFDA plasma concentrations were below the LOD for 44% of maternal samples). We also examined the associations of PFOS and PFOA isomers with detectable concentrations (i.e., n-PFOA, n-PFOS, Sm-PFOS) with aBMD z-score. Additionally, we evaluated the association between plasma PFAS concentrations and BMC z-score. While BMC does not track as strongly as BMD with its z-score at skeletal maturity (Wren et al. 2014), it incorporates bone mass (g) and, compared with BMD, has the advantage of accounting for bone thickness and may be less susceptible to confounding due to bone size (Crabtree et al. 2014). Finally, we assessed for effect modification by child sex via an interaction term and stratification.
For penalized spline generalized additive models and WQS regression, we used R (version 3.3.2; R Development Core Team), and for all other analyses, SAS (version 9.4; SAS Institute Inc.).
Results
Population Characteristics
Children were [] of age at the midchildhood visit, and () aBMD z-score was . Forty-nine percent of children were female, 57% were white, and mothers of 64% were college graduates at the time of cohort enrollment. Children with the highest PFOA concentrations were more likely to be younger, white, live in a census tract with higher median household income, and to have greater dairy intake and earlier blood collection. Mothers of children with the highest PFOA concentrations were more likely to be older and college graduates (Table 1).
Table 1.
Participant characteristics overall () and by quartiles of total perfluorooctanoic acid (PFOA) plasma concentration in midchildhood.
| Overalla | Quartiles of PFOAb | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| or n (%) | |||||
| Maternal/neighborhood characteristics | |||||
| Maternal age at enrollment (y) | |||||
| College graduate (%) | 364 (64) | 59 (41) | 89 (61) | 101 (73) | 115 (80) |
| Individual household income (%) | |||||
| 85 (16) | 39 (30) | 20 (14) | 15 (11) | 11 (8) | |
| 89 (16) | 25 (19) | 23 (17) | 22 (16) | 19 (13) | |
| 369 (68) | 65 (51) | 96 (69) | 98 (73) | 110 (79) | |
| Census tract median household income (%)c | |||||
| 35 (6) | 15 (11) | 12 (8) | 4 (3) | 4 (3) | |
| 248 (44) | 84 (59) | 61 (42) | 57 (41) | 46 (32) | |
| 244 (43) | 39 (27) | 64 (44) | 71 (51) | 70 (49) | |
| 43 (7) | 4 (3) | 8 (6) | 8 (6) | 23 (16) | |
| Child characteristics | |||||
| Age (y) | |||||
| Female (%) | 280 (49) | 73 (50) | 73 (50) | 101 (45) | 71 (49) |
| Race/ethnicity (%) | |||||
| White | 328 (57) | 37 (26) | 80 (54) | 94 (68) | 117 (81) |
| Black | 129 (23) | 66 (46) | 32 (22) | 21 (15) | 10 (7) |
| Asian | 14 (2) | 6 (4) | 2 (1) | 2 (1) | 4 (3) |
| Hispanic | 34 (6) | 13 (9) | 13 (9) | 6 (4) | 2 (1) |
| Other | 69 (12) | 22 (15) | 20 (14) | 16 (12) | 11 (8) |
| Dairy intake (servings per week) | |||||
| Physical activity (hours per week) | |||||
| Year of blood draw (%)c | |||||
| 2007 | 64 (11) | 6 (4) | 17 (12) | 16 (11) | 25 (17) |
| 2008 | 203 (35) | 21 (14) | 39 (27) | 62 (44) | 81 (56) |
| 2009 | 189 (33) | 56 (39) | 58 (39) | 45 (32) | 30 (21) |
| 2010 | 120 (21) | 62 (43) | 33 (22) | 17 (12) | 8 (6) |
| aBMD z-score | |||||
| BMC z-score | |||||
Note: aBMD, areal bone mineral density; BMC, bone mineral content; Q1, first/lowest quartile; SD, standard deviation.
Missing data for participants overall (): 4 missing maternal education, 6 census tract median household income, 33 individual household income, 2 race/ethnicity, 22 dairy intake, and 22 physical activity.
PFOA quartile minimum and maximum values: limit of detection for Q1, for Q2, for Q3, and for Q4.
Percentages do not add up to 100% due to rounding.
Most PFASs considered in our study (except PFDA and MeFOSAA) were detectable in the plasma of of children. Plasma PFAS concentrations in our study were similar to those reported in U.S. children in the National Health and Nutrition Examination Survey (NHANES) during the same period, from 2007 to 2008 (CDC 2018). The highest PFAS plasma concentrations in Project Viva were of PFOA [median (IQR) ] and PFOS [median (IQR) ]. PFAS plasma concentrations were moderately correlated [Spearman’s rank order correlation coefficient ], with the strongest correlations between PFOS and PFOA () (see Table S3).
Single-Per- and Polyfluoroalkyl Substance Models
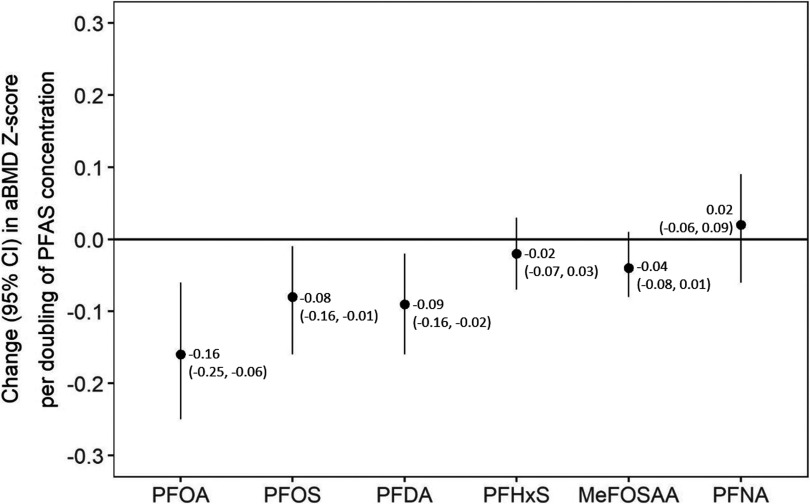
Children with higher plasma PFAS concentrations had lower aBMD z-scores in covariate-adjusted models (Figure 1), with strongest associations for PFOA (: ; 95% CI: , per doubling of PFOA), PFOS ( ; 95% CI , per doubling of PFOS), and PFDA ( ; 95% CI , per doubling of PFDA). Each doubling of MeFOSAA was associated with 0.04 unit lower aBMD z-score [95% confidence interval (CI): , 0.01], but CIs included the null. PFHxS and PFNA were not associated with aBMD z-score.
Figure 1.
Single-per- and polyfluoroalkyl substance (PFAS) models showing adjusted associations of individual PFAS plasma concentrations with areal bone mineral density (aBMD) z-score. Note: Adjusted for maternal age, education, census tract median household income, individual household income, and child age, sex, race/ethnicity, year of blood draw, dairy intake, and physical activity. for all single-PFAS models. CI, confidence interval; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamide) acetate; PFNA, perfluorononanoate.
Multi-Per- and Polyfluoroalkyl Substance Model
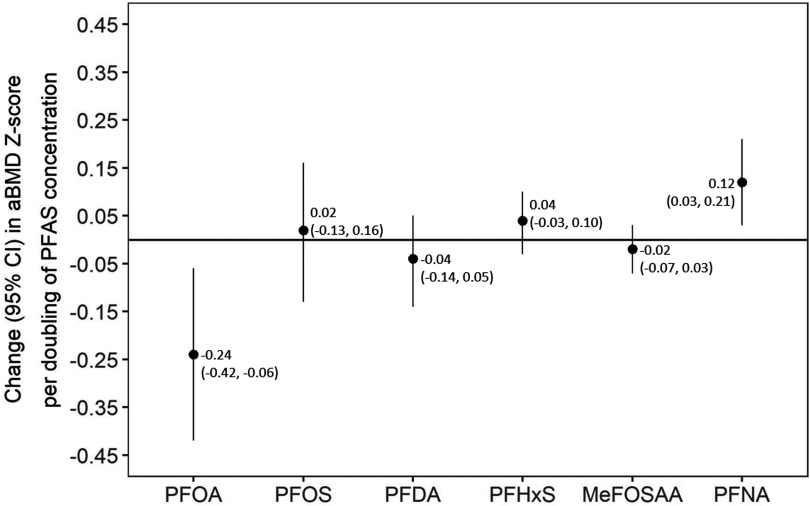
In the covariate-adjusted multi-PFAS model, each doubling of PFOA was associated a 0.24-unit-lower aBMD z-score (95% CI: , ), whereas each doubling of PFNA was associated with a 0.12-unit-higher aBMD z-score (95% CI: 0.03, 0.21) (Figure 2). Other PFASs were not associated with aBMD z-score.
Figure 2.
Multi-per- and polyfluoroalkyl substance (PFAS) model showing adjusted associations of PFAS plasma concentrations with areal bone mineral density (aBMD) z-score. Note: Adjusted for maternal age, education, census tract median household income, individual household income, and child age, sex, race/ethnicity, year of blood draw, dairy intake, and physical activity. . CI, confidence interval; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; MeFOSAA - 2-(N-methyl-perfluorooctane sulfonamide) acetate; PFNA, perfluorononanoate.
Weighted Quantile Sum Regression Model
The WQS index was negatively associated with aBMD z-score. Each IQR increment in the WQS index was associated with a lower aBMD z-score (95% CI: , ). Within the mixture, PFDA had the highest weight (37%), followed by MeFOSAA (29%). As a result of their strong correlation with each other (), PFOA and PFOS had lower individual weights (23% and 8%, respectively). However, in combination, PFOS and PFOA contributed 31% of the total strength of the association between the PFAS mixture and aBMD z-score. Weights of PFHxS (3%) and PFNA (0%) were lower.
Sensitivity Analyses
When we adjusted single-PFAS models for maternal plasma concentrations of each PFAS in early pregnancy, associations between midchildhood plasma PFAS concentrations and aBMD z-score were slightly stronger. For example, among participants with data on maternal PFOA plasma concentration (), per doubling of PFOA, aBMD z-score was 0.18 units lower (95% CI: , ) adjusting for maternal PFOA plasma concentration, vs. 0.16 units lower (95% CI: , ) without adjustment (see Table S4).
The isomers of PFOA and PFOS we considered in this study had modestly stronger covariate-adjusted associations with aBMD z-score than their parent compounds. Areal BMD z-score was 0.23 units lower (95% CI: , ) per doubling of n-PFOA vs. 0.16 units lower (95% CI: , ) per doubling of total PFOA. Similarly, aBMD z-score was 0.12 units lower (95% CI: , ) per doubling of n-PFOS and 0.12 units lower (95% CI: , ) per doubling of Sm-PFOS vs. 0.08 units lower (95% CI: , ) per doubling of total PFOS.
The directionality of associations between PFAS plasma concentrations and BMC z-score were similar to aBMD z-score in single-PFAS models, although CIs crossed the null for all PFAS–aBMC z-score associations (see Figure S2). For example, BMC z-score was 0.08 units lower (95% CI: , 0.01), whereas aBMD z-score was 0.16 units lower (95% CI: , ) per doubling of PFOA.
In single-PFAS models, no associations between plasma PFAS concentrations and aBMD z-score were modified by child sex (p-interaction terms were all ). However, we observed stronger associations of PFOA and PFDA plasma concentrations with aBMD z-score in girls as compared with boys in stratified analyses. In girls, aBMD z-score was 0.18 units lower (95% CI: , ) per doubling of PFOA [vs. 0.09 units lower (95% CI: , 0.02) in boys, ] and 0.13 units lower (95% CI: , ) per doubling of PFDA [vs. 0.04 lower (95% CI: , 0.05) in boys, ] (see Table S4).
Discussion
Our analysis of data from a large Boston-area cohort is among the first to investigate the role of an environmental exposure on bone mineral density in childhood. We found that children with greater plasma concentrations of select PFASs had lower aBMD z-scores during midchildhood.
Our findings align with our a priori hypothesis that higher PFAS exposure would be associated with lower BMD in childhood and are consistent with established biological pathways of action. For example, PFASs have been shown to activate (Vanden Heuvel et al. 2006), which triggers mesenchymal stem cells to differentiate into adipocytes at the expense of osteoblasts and may thereby reduce BMD (Marciano et al. 2015; Yamamoto et al. 2015). PFASs also act as androgen receptor antagonists (Kjeldsen and Bonefeld-Jorgensen 2013), which may further lower BMD (Clarke and Khosla 2009). In addition, animal models suggest PFOS at environmentally relevant doses rapidly deposits into bone tissue (Bogdanska et al. 2011). In a study of human bone bank and cadaver samples, PFOA and PFOS were sequestered in all femoral head samples and detected in bone marrow, with an association between higher bone PFOS concentration and lower bone volume, although this result was not statistically significant (Koskela et al. 2017). Our findings substantiate existing evidence of biological pathways through which PFAS exposure may lower aBMD z-score.
Our research confirms and extends one relatively small study in children and two studies in older adults that found a cross-sectional association between greater PFAS plasma concentrations and lower BMD. In a pilot study of 48 obese children aged 8–12 y, greater plasma concentrations of PFOA, PFOS, PFNA, and PFHxS were associated with poorer bone health (Khalil et al. 2018). However, this study was limited by a small sample size, limited statistical precision, and the use of calcaneal ultrasound bone measurements, which is not a standard bone assessment tool for use in children (Gordon et al. 2014). Similarly, in 2,339 NHANES adults, greater PFOS but not PFOA was associated with lower lumbar spine BMD in premenopausal women but not postmenopausal women or men (Lin et al. 2014). In a different study of 1,914 NHANES adolescents and adults, higher serum PFOS concentrations were associated with lower femoral neck BMD in men, and higher serum PFOS and PFOA concentrations were associated with lower femur BMD in women (Khalil et al. 2016). Thus, our finding of an association between greater PFAS plasma concentrations and lower BMD are in line with the limited existing epidemiologic literature.
To put our findings into perspective, the magnitude of the association between PFAS exposure and aBMD z-score in the present study [0.16-unit-lower aBMD z-score (95% CI: , ) per IQR increment in the WQS index] was greater than that of other well-known determinants of aBMD evaluated in the Bone Mineral Density in Childhood Study (Kalkwarf et al. 2007). For example, spine aBMD z-score was 0.04 higher (95% CI: 0.02, 0.06) per additional hour per day of high-impact physical activity and lower (95% CI: , ) per 1% increase in a BMD genetic risk score (Mitchell et al. 2016). Future studies of PFAS exposure and fracture incidence will help to elucidate the clinical relevance of these findings.
An advantage of our analysis was our ability to examine associations of several individual PFASs and the entire mixture with aBMD z-score. In single-PFAS models, we observed plasma concentrations of PFOA, PFOS, and PFDA to be most strongly associated with lower aBMD z-score. When we examined associations of individual PFASs independent of the other PFASs in a multi-PFAS model, only PFOA remained significantly associated with lower aBMD z-score. In our mixture model, we found the association between the PFAS mixture and lower aBMD z-score to be driven by PFDA. The different results may be due to high collinearity between PFOS and PFOA, as the mixture model accounts for collinearity between compounds, while the multi-PFAS model does not. It is important to note that PFDA plasma concentrations had relatively small variability in our cohort, with a range of . Few epidemiologic studies have evaluated PFDA, and additional research would increase our understanding of the potential role of PFDA on aBMD and other health outcomes. Overall, our findings are in line with prior studies in our cohort that have shown PFOA, PFOS, and PFDA to have the strongest associations with other health outcomes, including insulin resistance (Fleisch et al. 2017), lipid profile (Mora et al. 2018), and visual–motor abilities (Harris et al. 2018).
Interestingly, in our multi-PFAS model, we found PFNA to be associated with higher (rather than lower) aBMD z-score. A similar inference may be made from the results of our WQS model, in which PFNA received a weight of zero in the composite weighted index, indicative of no (or negligible) negative association (Carrico et al. 2015). This finding is consistent with the fact that PFNA activates more strongly than the other PFASs (Vanden Heuvel et al. 2006; Wolf et al. 2010), and activation stimulates bone development and increases aBMD (Stunes et al. 2011). Simultaneous activation of and may reduce the negative bone effects of alone (Smith et al. 2012). The opposite direction of effect observed for PFNA in relation to aBMD z-score in our study, when compared with other PFAS, supports existing evidence of variation in mechanisms of action between PFASs and their consequent association with health outcomes.
We observed a stronger association of PFASs with aBMD in females than males, although these differences were not statistically significant. The direction and magnitude of our results are consistent with findings from the two prior adult NHANES analyses in which PFAS serum concentrations were associated with lower aBMD (Khalil et al. 2016; Lin et al. 2014) and higher osteoporosis risk (Khalil et al. 2016) in females relative to males. Rodent studies have also shown differences in the pharmacokinetic characteristics and tissue distribution of PFASs (specifically PFOA, PFOS, and PFHxS), with more rapid absorption in female as compared with male rats (Kim et al. 2016). Our study results align with these observations and strengthen the case for investigating sex-based differences in biological PFAS activity in humans.
As far as we are aware, our study is the largest data set to date to examine the association between childhood PFAS exposures (estimated from PFAS plasma concentrations) and aBMD, and the only pediatric study to evaluate total body (excluding skull) aBMD z-score using DXA, which is widely considered the gold standard for pediatric bone density measurements (Gordon et al. 2014). A particular strength of this study was our evaluation of several PFASs and our ability to consider multiple potential confounding factors, including prenatal PFAS exposure. We were also able to account for determinants of bone mass, such as dairy intake and physical activity, although physical activity is challenging to quantify in children (Rowlands and Eston 2007) and limited by questionnaire measures in this cohort. In addition, we used WQS regression, a novel statistical approach to address highly correlated mixtures of exposures. A limitation of our cross-sectional analysis is that we were not temporally positioned to assess for mediation by pubertal status or BMI, which have both been associated with PFAS plasma concentration (Lopez-Espinosa et al. 2011; Domazet et al. 2016) and aBMD (Rokoff et al. 2019; Cousminer et al. 2018). In addition, our sample size was not considered robust to splitting into training and validation data sets in the WQS analysis, and therefore, weights were estimated in the same data used to test for significance. Our results may have been influenced by multiple testing for associations between several PFAS and BMD, although consistent patterns in our results reduce this concern.
In summary, we are among the first to evaluate environmental exposures in relation to bone health in childhood. We observed higher exposure to PFASs to be associated with lower aBMD z-scores in children. While replication of our findings is necessary, lower exposures to environmental toxicants such as PFASs during childhood may improve childhood bone accrual and thereby optimize peak bone mass and lifelong skeletal health.
Supplementary Material
Acknowledgments
We thank the staff and participants of Project Viva for their support. We also acknowledge K. Kato, A. Patel, and T. Jia for technical assistance in measuring the PFASs.
The authors have received support from the National Institutes of Health (R01ES021447, R01HD034568, K23ES024803, UG3OD023286, R01ES030101). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4918).
These authors contributed equally to this work.
Deceased.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Baker AM, Haeri S, Camargo CA, Stuebe AM, Boggess KA. 2011. A nested case-control study of first-trimester maternal vitamin D status and risk for spontaneous preterm birth. Am J Perinatol 28(9):667–672, PMID: 21500145, 10.1055/s-0031-1276731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanska J, Borg D, Sundström M, Bergström U, Halldin K, Abedi-Valugerdi M, et al. . 2011. Tissue distribution of 35s-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology 284(1–3):54–62, PMID: 21459123, 10.1016/j.tox.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Brownbill RA, Ilich JZ. 2005. Measuring body composition in overweight individuals by dual energy x-ray absorptiometry. BMC Med Imaging 5(1):1, PMID: 15748279, 10.1186/1471-2342-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES). Environ Sci Technol 41(7):2237–2242, PMID: 17438769, 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20(1):100–120, PMID: 30505142, 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2018. National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2018. CDC, Atlanta, GA. [Google Scholar]
- Clarke BL, Khosla S. 2009. Androgens and bone. Steroids 74(3):296–305, PMID: 18992761, 10.1016/j.steroids.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. . 2010. Illustrating bias due to conditioning on a collider. Int J Epidemiol 39(2):417–420, PMID: 19926667, 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousminer DL, Mitchell JA, Chesi A, Roy SM, Kalkwarf HJ, Lappe JM, et al. . 2018. Genetically determined later puberty impacts lowered bone mineral density in childhood and adulthood. J Bone Miner Res 33(3):430–436, PMID: 29068475, 10.1002/jbmr.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. . 2014. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 17(2):225–242, PMID: 24690232, 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. 2016. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart study. Diabetes Care 39(10):1745–1751, PMID: 27489335, 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. . 2017. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect 125(3):481–487, PMID: 27586368, 10.1289/EHP303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, et al. . 2011. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr 158(1):100–105, PMID: 20797727, 10.1016/j.jpeds.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CM, Leonard MB, Zemel BS. 2014. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 17(2):219–224, PMID: 24657108, 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Zemel BS, Wren T, Leonard MM, Bachrach LB, Rauch F, et al. . 2017. The determinants of peak bone mass. J Pediatr 180:261–269, PMID: 27816219, 10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Ye X, Bellinger DC, et al. . 2018. Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFASs) and child cognition. Environ Int 115:358–369, PMID: 29705692, 10.1016/j.envint.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, et al. . 2017. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6-10 year old American children. Environ Sci Technol 51(9):5193–5204, PMID: 28325044, 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. . 2007. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 92(6):2087–2099, 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, et al. . 2016. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. Population in NHANES 2009–2010. Environ Health Perspect 124(1):81–87, PMID: 26058082, 10.1289/ehp.1307909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. . 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8-12 year old children: a pilot study. Environ Res 160:314–321, PMID: 29040951, 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Heo SH, Lee DS, Hwang IG, Lee YB, Cho HY. 2016. Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food Chem Toxicol 97:243–255, PMID: 27637925, 10.1016/j.fct.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jorgensen EC. 2013. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20(11):8031–8044, PMID: 23764977, 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, Tuukkanen J. 2017. Perfluoroalkyl substances in human bone: concentrations in bones and effects on bone cell differentiation. Sci Rep 7(1):6841, PMID: 28754927, 10.1038/s41598-017-07359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LY, Wen LL, Su TC, Chen PC, Lin CY. 2014. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in U.S. premenopausal women: NHANES, 2005–2008. J Clin Endocrinol Metab 99(6):2173–2180, PMID: 24606077, 10.1210/jc.2013-3409. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. . 2011. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45(19):8160–8166, PMID: 21534542, 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- Marciano DP, Kuruvilla DS, Boregowda SV, Asteian A, Hughes TS, Garcia-Ordonez R, et al. . 2015. Pharmacological repression of PPARγ promotes osteogenesis. Nat Commun 6:7443, PMID: 26068133, 10.1038/ncomms8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Chesi A, Elci O, McCormack SE, Roy SM, Kalkwarf HJ, et al. . 2016. Physical activity benefits the skeleton of children genetically predisposed to lower bone density in adulthood. J Bone Miner Res 31(8):1504–1512, PMID: 27172274, 10.1002/jbmr.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, et al. . 2018. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int 111:1–13, PMID: 29156323, 10.1016/j.envint.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. . 2015. Cohort profile: Project Viva. Int J Epidemiol 44(1):37–48, PMID: 24639442, 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D, et al. . 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362, PMID: 23892228, 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Burlet N. 2006. Osteoporosis: a still increasing prevalence. Bone 38(2 Suppl 1):S4–S9, PMID: 16455317, 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. 2001. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr 4(2):249–254, PMID: 11299098, 10.1079/PHN200061. [DOI] [PubMed] [Google Scholar]
- Rokoff LB, Rifas-Shiman SL, Switkowski KM, Young JG, Rosen CJ, Oken E, et al. . 2019. Body composition and bone mineral density in childhood. Bone 121:9–15, PMID: 30557635, 10.1016/j.bone.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AV, Eston RG. 2007. The measurement and interpretation of children's physical activity. J Sports Sci Med 6(3):270–276, PMID: 24149412. [PMC free article] [PubMed] [Google Scholar]
- Rubin K, Schirduan V, Gendreau P, Sarfarazi M, Mendola R, Dalsky G. 1993. Predictors of axial and peripheral bone mineral density in healthy children and adolescents, with special attention to the role of puberty. J Pediatr 123(6):863–870, PMID: 8229518, 10.1016/s0022-3476(05)80381-6. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, et al. . 2015. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 49(19):11849–11858, PMID: 26333069, 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK. 2006. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91(8):3055–3061, PMID: 16720650, 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- Smith S, Samadfam R, Chouinard L, Awori M, Benardeau A, Bauss F, et al. . 2012. Combined activation of PPAR-gamma and PPAR-alpha reduces the negative effects of PPAR-gamma agonism on bone and may attenuate PPAR-gamma-mediated loss of bone strength. Bone 50(Suppl 1):S164, 10.1016/j.bone.2012.02.513. [DOI] [Google Scholar]
- Stunes AK, Westbroek I, Gustafsson BI, Fossmark R, Waarsing JH, Eriksen EF, et al. . 2011. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord 11:11, PMID: 21615901, 10.1186/1472-6823-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000. US Census 2000: Summary File 3. http://www.census.gov/census2000/sumfile3.html [accessed 18 May 2015].
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92(2):476–489, 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Zehr RD, Schmid JE, Lau C, Abbott BD. 2010. Developmental effects of perfluorononanoic acid in the mouse are dependent on peroxisome proliferator-activated receptor-alpha. PPAR Res 2010:1, 10.1155/2010/282896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, et al. . 2014. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr 164(6):1280–1285, PMID: 24485819, 10.1016/j.jpeds.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Yamane T, Oishi Y, Kobayashi-Hattori K. 2015. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation in 3T3-l1 adipocytes. Biosci Biotechnol Biochem 79(4):636–639, PMID: 25516096, 10.1080/09168451.2014.991683. [DOI] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong LY, Jia T, Kalathil A, Latremouille J, et al. . 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health 221(1):9–16, PMID: 28993126, 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. . 2011. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96(10):3160–3169, PMID: 21917867, 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Hu GX, Chu Y, Jin X, Gong S, Akingbemi BT, et al. . 2010. Inhibition of human and rat 3-beta-hydroxysteroid dehydrogenase and 17-beta-hydroxysteroid dehydrogenase 3 activities by perfluoroalkylated substances. Chem Biol Interact 188(1):38–43, PMID: 20619251, 10.1016/j.cbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.