Fig. 1.
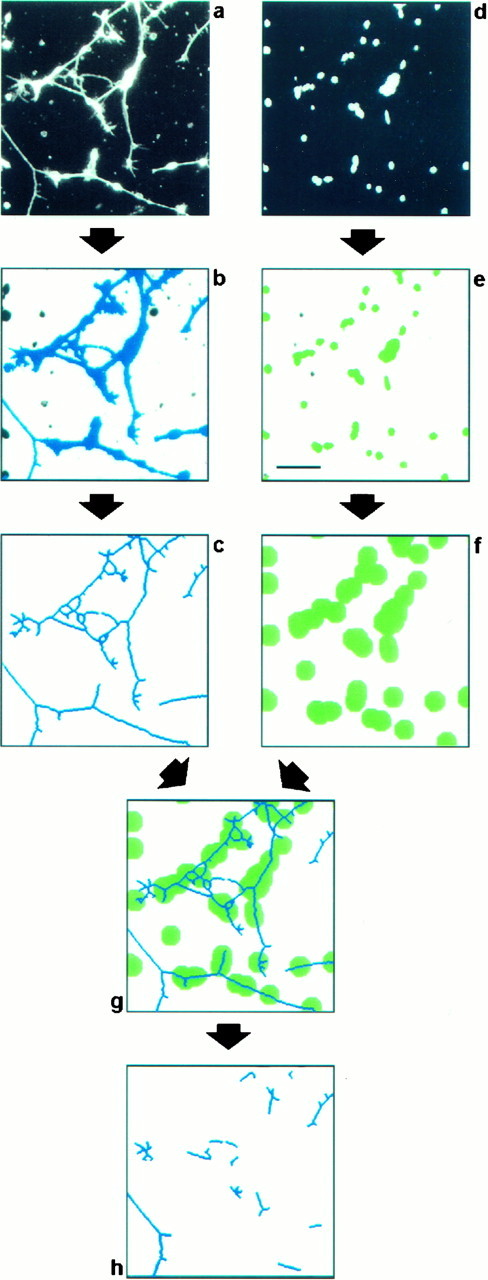
Digital image analysis procedure to evaluate neurite outgrowth in vitro. Retinal cells were seeded on a substratum composed of a mixture of FN and F11. After cultivation for 20 hr, cell bodies and processes were detected by immunofluorescence analysis using antibodies directed to NCAM (a), and cellular nuclei were identified by bisbenzimide staining of DNA (d). To discriminate neurites from cell bodies, both fields were processed as follows. The field showing cells and neurites (a) was processed by applying a staining intensity threshold and size exclusion criteria to distinguish the significant structures representing neurons from background and from small artifactual signals of subcellular size (b, blue objects). These structures were then eroded to a skeleton of a single-pixel width (c). The field comprising cellular nuclei (d) was also processed by applying a staining intensity threshold and size exclusion criteria to label cellular nuclei (e, green objects). These were used to estimate the number of attached cells by dividing the total area representing cell nuclei by the average area covered by a single nucleus. Because we are interested in quantifying primarily the long neurites with a length of more than two cell diameters, all structures within this distance of every cell nucleus had to be excluded from the analysis. To this end, every object that represents a cell nucleus was enlarged correspondingly (f). A comparison of the resulting image with the image representing putative neurites (c) allows the identification of long neurites and fragments thereof, because they do not overlap with the enlarged nuclei (g). To enhance the stringency of the procedure further, objects below a defined length threshold were discarded. The remaining objects (h) were counted, and their individual lengths were measured automatically. The values obtained were normalized with respect to the cell number to correct for intra-assay variations in the number of attached cells. In this procedure we did not attempt to measure the real number of neurites in the observed field or their real length, but we were merely interested in parameters that reflect the outgrowth and can be used to quantify the response of a large number of neurons. Scale bar, 50 μm.
