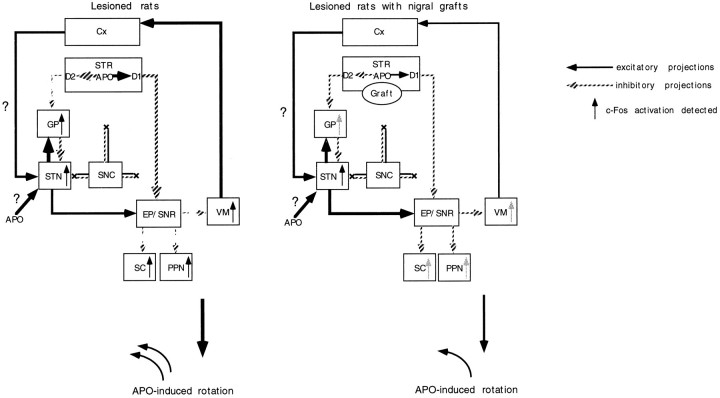
Fig. 9.
Diagrammatic representation of a hypothetical model of a cascade of changes of neuronal activity in the basal ganglia after systemic injections of apomorphine (modified from Albin et al., 1989). After apomorphine (APO) challenge, the activity of striatopallidal GABAergic neurons with hypersensitive D2 receptors is suppressed, with consequent disinhibition of the globus pallidus (GP). Apomorphine could directly induce c-Fos activation in the DA-denervated subthalamic nucleus (STN). The lesion-induced DA denervation of the neocortex (Cx) could also affect the excitability of the STN to cause the c-Fos activation in the STN after injections of apomorphine. Stimulation of striatal D2 receptors with supersensitive state may suppress the activity of the entopeduncular nucleus (EP) and substantia nigra pars reticulata (SNR) through an inhibition of the activity of glutamatergic STN neurons (indirect pathway). The suppression of the activity of the EP and SNR can lead to disinhibition of the activity in their target structures, such as the ventromedial thalamic nucleus (VM), superior colliculus (SC), and pedunculopontine nucleus (PPN). Stimulation of striatal D1 receptors, which directly suppresses the neuronal activity in the EP and SNR (direct pathway), also leads to bursting activity in the VM, SC, and PPN by a disinhibitory mechanism. The graft-mediated reversal of the increased levels of striatal D1 and D2 receptors can partly reverse the altered activity of basal ganglia circuitry to attenuate the apomorphine-induced bursting activity of the GP as well as the basal ganglia target structures.