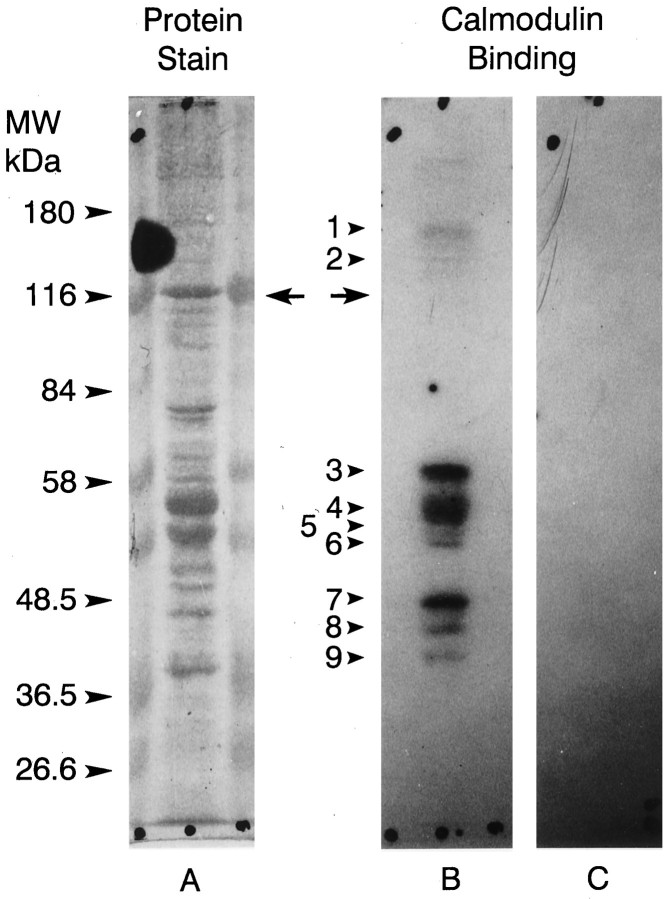
Fig. 8.
125I-Calmodulin binding to Western blots of ventral photoreceptor cell body proteins. Ventral photoreceptor cell bodies dissected from two animals were pooled, homogenized (Edwards and Battelle, 1987), fractionated by SDS-PAGE on 7.5% gels, and blotted to nitrocellulose as described in Materials and Methods. A, Fast green stain of one lane of the blot.B, Autoradiograph of the same lane shown inA incubated with 125I-calmodulin plus 1 mm Ca2+. C, Autoradiograph of a duplicate lane incubated with125I-calmodulin with no added Ca2+. The locations of the molecular mass standards are indicated. Thearrows show where myoIIILim migrates. No calmodulin binding was observed in the absence of Ca2+. The protein bands that bound calmodulin in the presence of Ca2+ are indicated witharrows and numbered. Their apparent molecular masses in kilodaltons are as follows: 1, 150;2, 133; 3, 57.5; 4, 52;5, 49; 6, 47.5; 7, 42;8, 40; 9, 37.5. MyoIIILim did not bind 125I-calmodulin in the presence or absence of Ca2+.