Fig. 9.
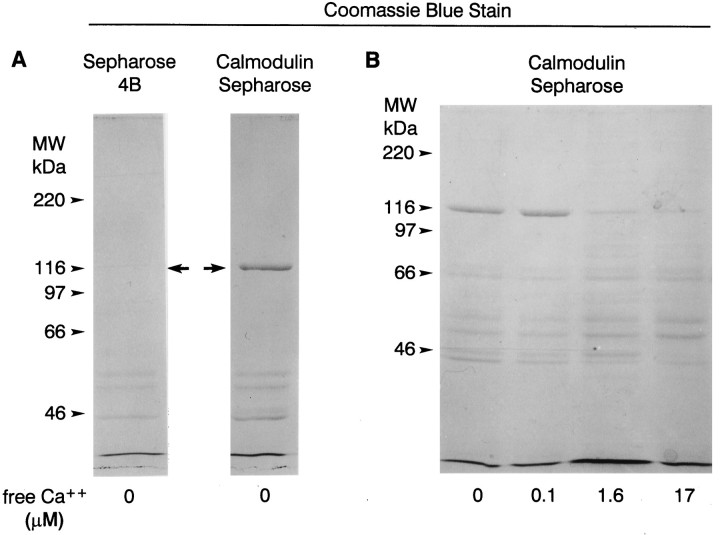
Coomassie blue-stained polyacrylamide gels showing proteins that bound to calmodulin-Sepharose and Sepharose 4B in the presence of different concentrations of Ca2+. Soluble extracts of Limulus lateral eye plus lateral optic nerves were incubated with calmodulin-Sepharose or Sepharose 4B without bound calmodulin in the absence or presence of different concentrations of Ca2+ (see Materials and Methods). Proteins that bound to the beads were extracted into SDS sample buffer, fractionated by SDS-PAGE, and stained with Coomassie blue. The positions of the molecular mass standards are indicated.A, In the absence of calcium, myoIIILim, the protein band that migrates close to the 116 kDa molecular mass standard (arrows), bound to calmodulin-Sepharose but not to Sepharose 4B. B, MyoIIILim bound to calmodulin-Sepharose in the absence of Ca2+ and in the presence of 0.1 μmfree Ca2+. Binding of myoIIILim to calmodulin-Sepharose was reduced in the presence of 1.6 and 17 μm free Ca2+, and the binding of other proteins was enhanced. Note in particular the bands that migrate at 57 and 53 kDa.