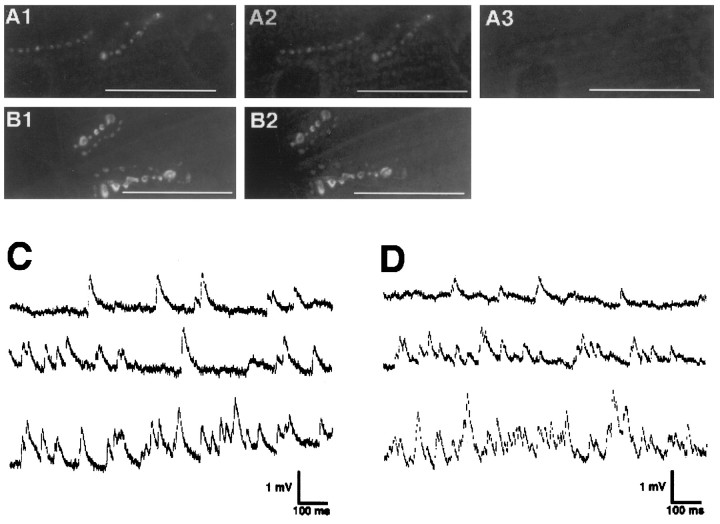
Fig. 3.
Calcimycin induces FM1-43 destaining and elevated quantal release in csp mutants at >30°C. A, NMJs of homozygouscspE16 mutant larvae were stained with FM1-43 by K+ depolarization at 22°C (data not shown), and the ts block of FM1-43 destaining was confirmed. At 32°C K+ stimulation failed to destain the labeled synaptic boutons (A1). Calcimycin (A23187) stimulation of FM1-43 destaining in calcium-free medium at 32°C released little, if any, FM1-43 dye from the synaptic boutons (A2). However, calcimycin stimulation in the presence of external Ca2+ at 32°C destained the same NMJ (A3) that failed to destain with the previous two stimuli (A1, A2). The images represent five independent preparations. B, After FM1-43 staining at 22°C (data not shown) and confirming the ts block of evoked FM1-43 destaining at 32°C (B1), NMJs of homozygouscspE16 mutants were treated for 5 min with 10 μm BAPTA-AM ester in calcium-free medium. After removal of the residual external BAPTA ester, subsequent calcimycin stimulation failed to destain the FM1-43-labeled and BAPTA-loaded synaptic boutons at 32°C (B2). The images represent five preparations. C, D, MEJPs were recorded contin-uously at 31°C in the presence of 0.5 mm Ca2+ and 1 μg/ml TTX from muscle fiber 6 of wild-type (C) andcspX1 mutant (D) larvae. The first trace (C, D) represents MEJP recordings before the application of calcimycin. Trace 2 shows the onset of the response (C, D) shortly after the application (30 sec) of calcimycin. Trace 3shows the highest activity, which was on average 42 ± 3 Hz (mean ± SEM; n = 3) for wild-type and 41 ± 5 Hz (mean ± SEM; n = 3) forcspX1 mutant larvae recorded before fast muscle depolarizations hindered any further electrophysiological analysis. Muscle potentials were −67 mV for wild-type (C) and −57 mV forcspX1 mutant (D) larvae. Scale bars, 50 μm.