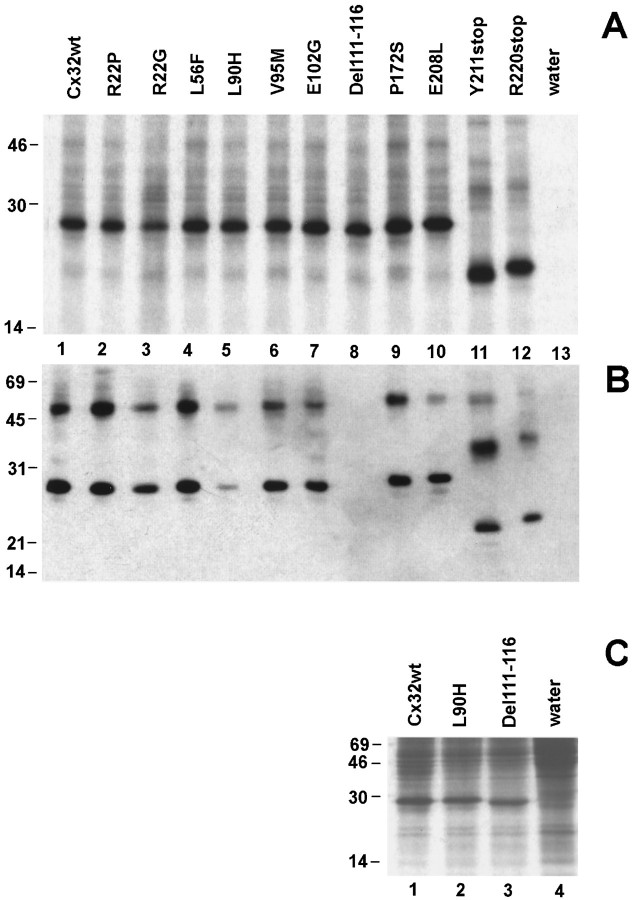
Fig. 2.
CMTX mutations of Cx32 are expressed byXenopus oocytes injected with in vitrotranscribed cRNAs. A, In vitrotranslation of cRNAs of Cx32wt and CMTX mutations. Each cRNA directed the synthesis of a major polypeptide product that, in the case of the two mutations predicting a premature stop codon, migrated with the expected faster electrophoretic mobility. B, Western blots of Xenopus oocytes membranes. In oocytes that received cRNAs for either wild type or 10 of 11 CMTX mutations of Cx32, the antibody M12.13 (Goodenough et al., 1988) reacted with a major protein product that exhibited the expected SDS-gel mobility between 22 and 28 kDa. In addition, the antibody recognized a protein band with slower electrophoretic mobility, most likely a dimer of Cx32. No major proteins were detected in control, water-injected oocytes (lane 13), as well as in membranes prepared from oocytes injected with the Del111–116 mutation (lane 8).C, Metabolic labeling of Xenopus oocytes. Oocytes injected with cRNAs produced high levels of the encoded proteins (lanes 1–3), which were easily detected above the background of endogenous proteins synthesized by water-injected cells (lane 4). Experimental conditions are indicated at the top of each lane. The molecular mass (in kDa) and migration of protein standards are indicated on the left edge of each gel.