Fig. 7.
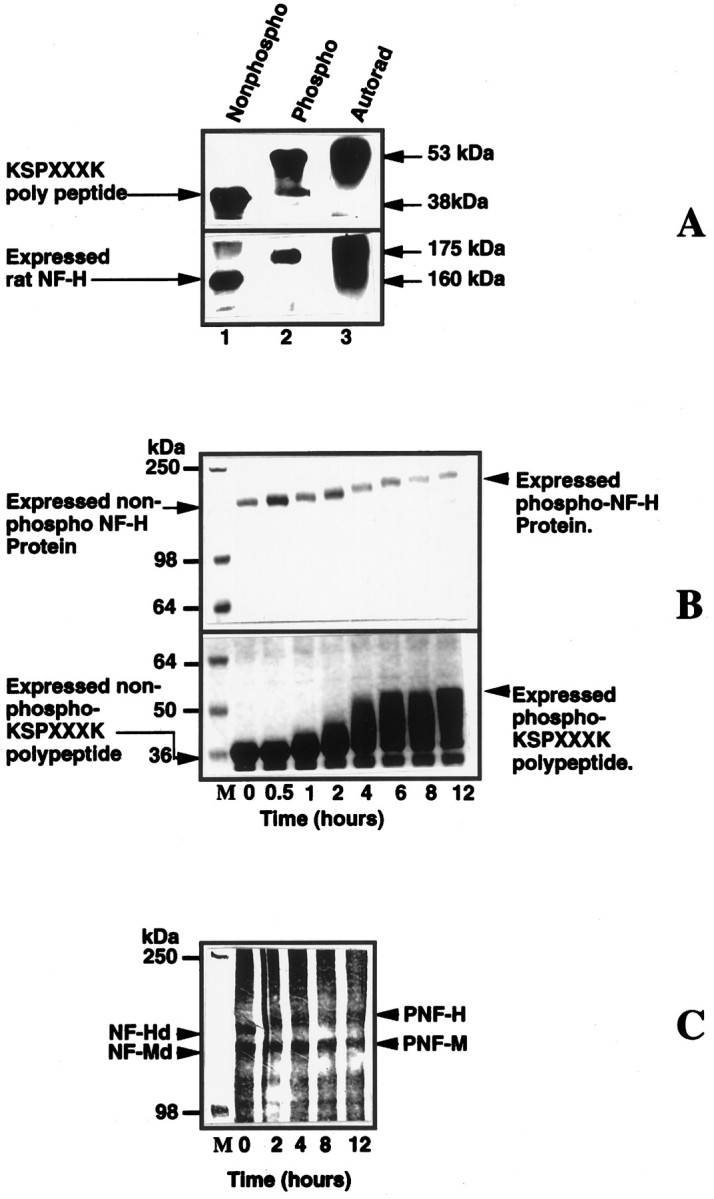
A mixture of Erk2 and MEK phosphorylate bacterially expressed rat NF-H, expressed NFH tail polypeptide (24 KSP repeats), and dephosphorylated native rat NF-H and NF-M.A, Phosphorylated substrates show a shift in electrophoretic mobility. Lanes 1 and 2are protein stains, and lane 3 is an autoradiogram oflane 2. Note the shift in the electrophoretic mobility of the phosphorylated substrates (lanes 2,3) as compared with the original unphosphorylated substrate in lane 1. B, Time course of the phosphorylation of expressed rat NF-H and expressed polypeptide. The kinase reaction was performed as described in Materials and Methods. The reaction was arrested at different time intervals by the (Figure legend continued) addition of Laemmli’s buffer, heated for 5 min, and loaded on to a 7.5% SDS gel; the proteins were visualized by staining with Coomassie blue stain. Note the time-dependent decrease in the electrophoretic mobility of the polypeptide and expressed NF-H as a function of increased phosphorylation. C, Time course of phosphorylation of native dephosphorylated NF-H and NF-M by Erk2. Both substrates exhibit decreased electrophoretic mobility after phosphorylation (proteins were visualized by silver staining after SDS-PAGE, using a 6% gel). The concentrations of MEK and Erk2 were 0.3 and 1.6 μg, respectively.
