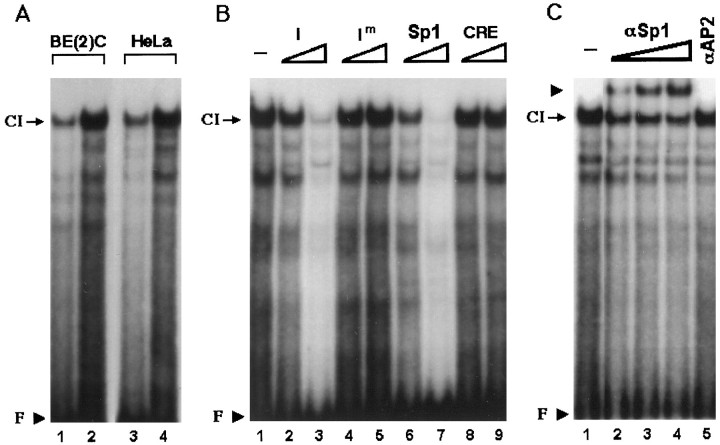
Fig. 3.
Domain I is an Sp1-binding site and interacts with the transcription factor Sp1 without cell type-specificity.A, 20 bp oligonucleotide containing domain I sequence was radiolabeled and incubated with 10 μg (lanes 1,3) or 20 μg (lanes 2,4) of SK-N-BE(2)C or HeLa nuclear extracts. A sequence-specific complex (CI) was formed by both nuclear extracts. Unbound free probe (F) is indicated by an arrowhead. B, Complex CI was competed by cold domain I (I) or Sp1 oligonucleotide but not by the mutant form of domain I (Im) or the CRE oligonucleotide, indicating that CI is a sequence-specific complex. For competition, 10-fold (lanes 2, 4, 6,8) or 100-fold (lanes 3,5, 7, 9) molar excesses of cold oligonucleotides were added to the reaction mixture containing 20 μg of SK-N-BE(2)c nuclear extracts before the addition of the radiolabeled probe. C, Twenty micrograms of SK-BE(2)C nuclear extracts were coincubated with 0.03 (lane 2), 0.1 (lane 3), or 0.3 (lane 4) μg of Sp1-specific antibody or with 0.1 μg (lane 5) of AP2-specific antibody. Coincubation with Sp1 antibody but not with AP2 antibody diminished formation of CI and resulted in formation of a supershifted band (arrowhead) in a dose-responsive manner.