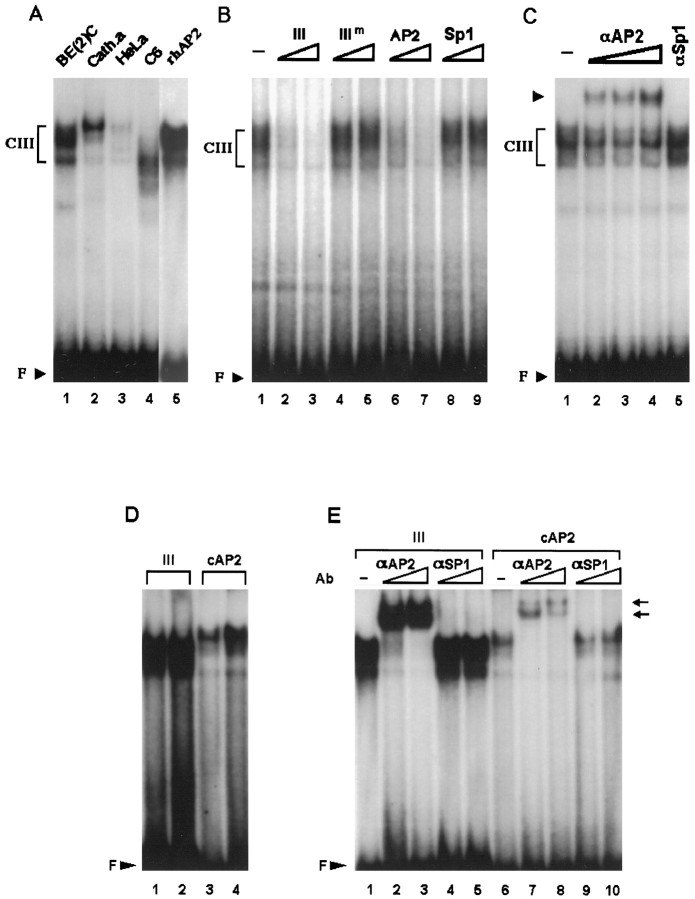
Fig. 6.
Domain III preferentially interacts with nuclear extracts from DBH-expressing cells and binds to the transcription factor AP2. A, 32P-labeled domain III oligonucleotide was incubated with 20 μg of nuclear extracts from each cell line as indicated at the top of thelanes. Three nanograms of the recombinant human AP2 protein (rhAP2) were also used in a control experiment (lane 5). B, CIII was competed by 40-fold (lanes 2, 4, 6,8) or 400-fold (lanes 3,5, 7, 9) molar excesses of domain III cold oligonucleotide or AP2 consensus sequence but not by the mutant form of domain III (IIIm) or Sp1 sequence. Twenty micrograms of SK-N-BE(2)C nuclear extracts were used for each binding reaction. C, In supershift assays, 0.03 (lane 2), 0.1 (lane 3), or 0.3 (lane 4) μg of AP2-specific antibody were used in each reaction along with 20 μg of SK-N-BE(2)C extracts. In a control experiment (lane 5), 0.1 μg of Sp1-specific antibody was used. D, Two nanograms (lanes 1, 3) or 10 ng (lanes 2,4) of recombinant AP protein is incubated with a32P-labeled domain III oligonucleotide (lanes 1, 2) or the consensus AP2 sequence (lanes 3, 4). AP2 protein bound to domain III sequence much more strongly than to the consensus sequence.E, Incubation of the recombinant AP2 protein with 0.03 (lanes 2, 7) or 0.1 (lanes 3, 8) μg of AP2-specific antibody completely diminished the specific complex and produced supershifted bands (arrows). In control experiments, 0.03 (lanes 4, 9) or 0.1 (lanes 5, 10) μg of Sp1-specific antibody did not affect the patterns of AP2-specific complexes formed with either domain III (lanes 4, 5) or the consensus AP2 sequence (lanes 9, 10).