Fig. 7.
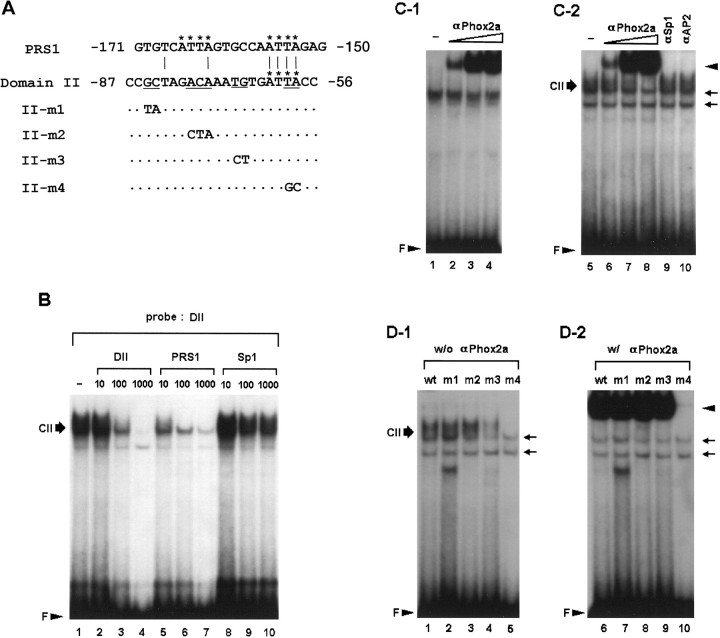
Domain II is another Phox2a-binding site.A, Nucleotide sequences of the PRS1 and wild-type and mutant of domain II oligonucleotides. The ATTA motifs are indicated byasterisks. Mutated bases are shown in the mutant oligonucleotides. Dots represent unchanged sequences.B, DNA–protein complexes formed with SK-N-BE(2)C nuclear proteins and the DII oligonucleotide were competed by molar excesses of different cold oligonucleotides as indicated above each panel. Thirty micrograms of nuclear proteins were used in each binding reaction. C, Antibody supershift assays indicate that Phox2a binds to both domain II and PRS1 sequences. Coincubation of nuclear proteins with increasing amounts of 1 μl of 10−2 (lanes 2,6), 10−1 (lanes 3, 7) and 1:3 dilution (lanes 4, 8) of Phox2a-specific antibody resulted in the generation of a supershifted band (arrowhead) in a dose-responsive manner with both PRS1 (C-1) and DII oligonucleotide (C-2). In addition, formation of domain II-specific complex, CII, was significantly diminished. In contrast, coincubation with either SP1 or AP2-specific antibody (0.1 μg each) neither generated the supershifted band nor diminished formation of CII (lanes 9, 10). Phox2a-specific antibody by itself was unable to form any complex with the radiolabeled probes (data not shown). D, Determination of nucleotide bases important for domain II-protein interaction in the absence (D-1) or presence (D-2) of Phox2a-specific antibody. m1 and m2 probes formed DNA–protein complexes as efficiently as the wild-type DII probe. m3 probe showed a limited ability to form complexes, and m4 probe shows the most severe defect for forming specific complexes (lanes 4, 5). In the presence of 1 μl of 1:3 dilution of Phox2a-specific antibody, even m3 was able to produce a robust band of supershifted complex as indicated by anarrowhead. m4 was able to generate little, if any, supershifted band, suggesting that the ATTA motif is critical for its interaction with Phox2a. Two nonspecific bands are indicated byarrows.