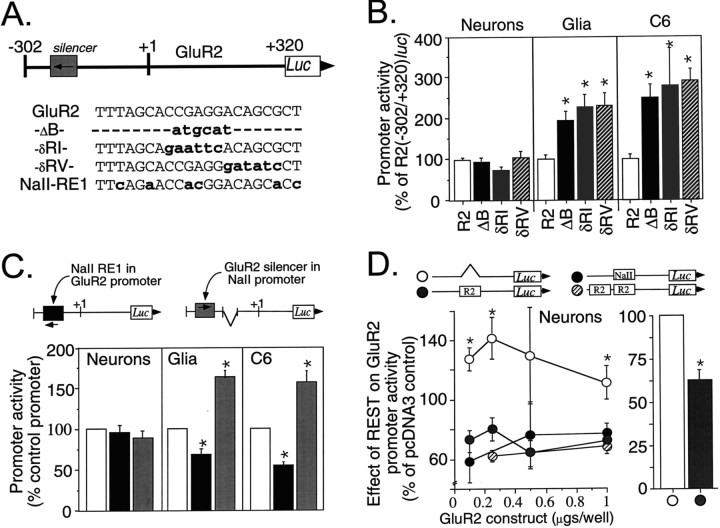
Fig. 6.
A weak GluR2 silencer interacts with the repressor REST. A, Schematic showing the location and antisense orientation of the silencer in the GluR2 promoter. The native (GluR2) and mutagenized silencer elements introduced into the R2(−302/+320)luc promoter construct are listed.Dashes represent deleted sequence, andlowercase, boldface type represents substitution mutations. The ΔB mutation is the same mutation as the internal deletion construct depicted in Figure 5. The NaII-RE1 sequence shown is the Type II Na channel RE1 silencer (Kraner et al., 1992), which differs from GluR2 in six positions. B, Functional analysis of silencer mutations on GluR2 promoter activity in primary cortical neurons, primary cortical glia, and C6 glioma cells. In neurons, no mutation resulted in a significant change in promoter activity from that of the R2(−302/+320)luc control. In primary glia and C6 glioma cells, all three silencer mutations resulted in two- to threefold increases in GluR2 promoter activity. Data shown are the mean ± SEM for 12–24 plasmid transfections in neurons, 14–22 plasmid transfections in glia, and 6–34 plasmid transfections in C6 gliomas from 10, 9, and 7 culture preparations, respectively. *p < 0.05 from the control by ANOVA andpost hoc Dunnett’s test. The error bar for the control (R2) represents the average SE among control plasmids. C, Promoter context independence of the GluR2 silencer. The GluR2 silencer was replaced by the NaII-RE1 silencer (seeA) in the context of the R2(−302/+320)luc promoter. Likewise, the NaII-RE1 silencer was replaced by the GluR2 silencer in the context of the NaII promoter construct. These constructs were transfected into primary neurons, glia, and C6 glioma cells. The NaII-RE1 silencer in the context of the GluR2 promoter (black bars) reduced promoter activity by 30–47% relative to that of the unmutated R2(−302/+320)luc control (open barsdefined as 100%) in glia and C6 glioma cells. In contrast, the GluR2 silencer in the context of the NaII promoter (gray bars) increased promoter activity by 54–61% relative to that of the unmutated NaII minimal promoter control (open bars also) in glia and C6 glioma cells. The effect of the alternative silencer in each of the promoters was significant from their respective controls; *p < 0.05, Student’st test. Data shown are the mean ± SEM for 8–9 plasmid transfections in neurons and 8–15 transfections in glia and C6 gliomas, all from three to five culture preparations for each cell type. D, Cotransfection of REST represses GluR2 promoter activity in neurons. The GluR2 silencer in the context of R2(−302/+320)luc was either unchanged (gray circles), deleted (open circles), replaced by the NaII silencer (black circles), or replaced by tandem GluR2 silencers (hatched circles) and then cotransfected into cultured cortical neurons with 0.75 μg of pCMV-RESTexpress per well or the pcDNA3 empty vector. The amount of R2(−302/+320)luc reporter plasmid was varied from 0.1 to 1.0 μg/well, and all tubes were balanced to 1.75 μg of total DNA/well with carrier DNA (pBluescript). REST cotransfection reduced GluR2 promoter activity by 20–42% from that of the pcDNA3 control in neurons in a silencer-dependent manner. This effect by REST was observed at all concentrations of input GluR2 promoter DNA tested (D, left). When luciferase activities were normalized to those of the silencer-deficient construct R2(−302/+320)luc-ΔB and pooled (right, open bar), REST coexpression reduced GluR2 promoter activity in neurons by an average of 37% (right, gray bar). Results are the mean ± SEM for 3–14 cotransfections in 14 culture preparations. Identical results were obtained using two independent preparations of pCMV-RESTexpress, pcDNA, and pBluescript plasmid DNA. *p < 0.05 or better from all other groups at the designated amount of input DNA (left, ANOVA and post hoc Dunnett’s) or from the silencer-deficient construct (right, Student’st test).