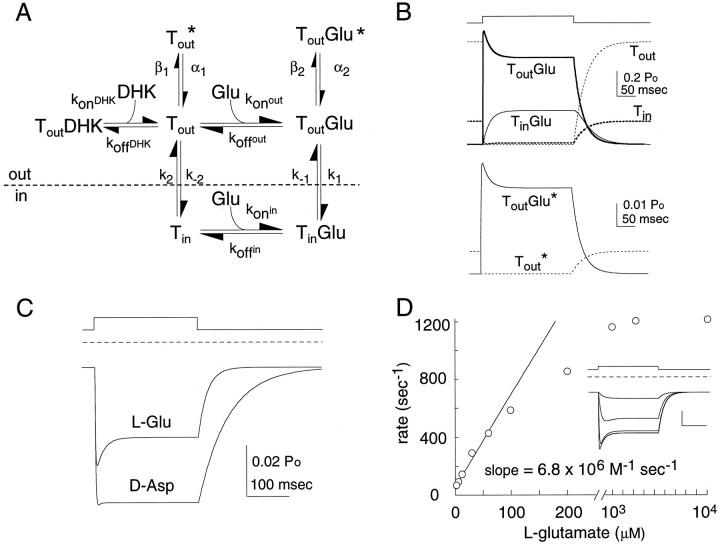
Fig. 8.
Computer simulation. A, Kinetic model of l-glutamate and d-aspartate transport and anion conductance. Model parameters were obtained by least squares fitting of data and fit an average pulse of l-glutamate ofd-aspartate. The microscopic rates were as follows:konout = 6.8 × 106m−1sec−1,koffout = 30.6 sec−1; k1 = 16.0 sec−1; k−1 = 2.9 sec−1;konin = 6.8 × 106m−1sec−1;koffin = 37.2 sec−1; k2 = 885 sec−1; k−2 = 200 sec−1; α1 = 8094 sec−1; β1 = 100 sec−1; α2 = 1260 sec−1; β2 = 70 sec−1. d-Aspartate data were fit with identical rates for agonist independent states andkoffout = 7.6 sec−1; k1 = 7.3 sec−1; k−1 = 1.0 sec−1;koffin = 165 sec−1; α2 = 978 sec−1, and β2 = 70 sec−1. DHK binding was assigned as 6.8 × 106m−1sec−1, whereas DHK unbinding (kdhkout) = 97 sec−1.B, Probability of occupancy for each state in the kinetic scheme shown in A during a 250 msec pulse of 10 mml-glutamate. The top traces show the nonconducting states: the unliganded states are represented bydashed lines (Tout andTin; bold), whereas the liganded states are represented by a solid line(ToutGlu; bold andTinGlu). The bottom traces show the occupancy of the anion conducting states. Note the different scale bars. C, Simulation of a 250 msec pulse of 10 mml-glutamate or d-aspartate (A). The channel’s steady-state open probability was determined from nonstationary noise analysis (Fig. 4) and the DHK-blocked currents (Fig. 6). The fraction of transporters in either conducting state TGluopen orTopen are plotted as a function of time.D, Concentration dependence of the time constant for activation of l-glutamate currents for the kinetic scheme shown in A. The time constants for the activation and deactivation were calculated by fitting the current records to a single exponential. The limiting slope for the activation rate equals 6.8 × 106m−1sec−1. Inset,l-Glutamate concentration dependence of the open probability (1 μm, 10 μm, 100 μm, and 1 mm). The model’s apparent affinity at steady state is 7 μm.