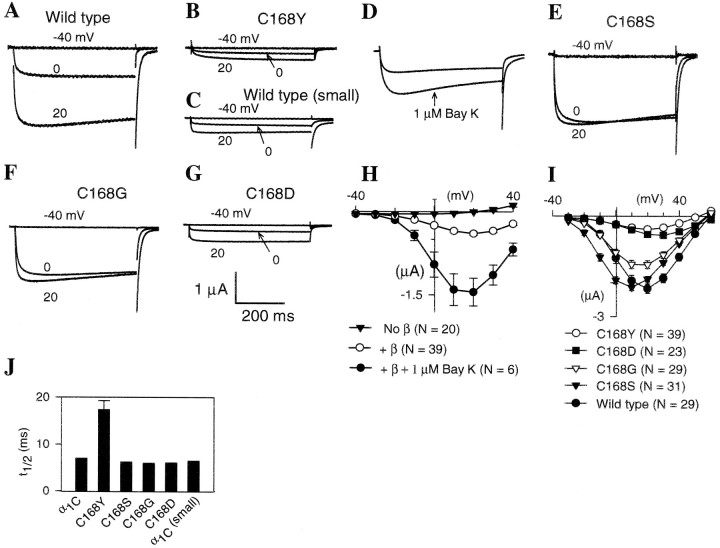
Fig. 3.
Functional characterization of amino acid substitutions introduced into the rabbit cardiac calcium channel α1CΔN60 subunit at the conserved Cys site in domain IS1. In the cardiac subunit, C168 is equivalent to C629 in theDrosophila Dmca1D α1 subunit. Oocytes were injected with 50 nl containing rabbit cardiac α1 and rat brain β1b. All oocytes were incubated for 3–4 d before recording, except those in C that were incubated only 1–2 d (A, C, rabbit cardiac α1CΔN60 subunit; B, E–G, mutant α1CΔN60 subunits with the Cys residue replaced as indicated). Currents shown were elicited from a holding potential of −80 mV using 500 msec voltage steps to −40, 0 and +20 mV.A–G, Representative current traces for oocytes expressing rabbit cardiac α1CΔN60 subunit variants. Oocytes were injected with truncated, wild-type α1CΔN60 (A, C) or one of the following mutations in α1CΔN60: C168Y (B, D), C168S (E), C168G (F), and C168D (G).D, Representative current traces from the mutant C168Y before (upper) and after (lower) treatment with 1 μm (−)-Bay K 8644. Vtest = 10 mV. H, Peak current versus test potential (I–Vcurves) for C168Y alone (filled inverted triangles), with β1 (open circles), and with β1 plus 1 μm(−)-Bay K 8644 (filled circles).I, Peak inward current versus test potential (I–V curves) for wild-type and mutant cardiac α1CΔN60 subunits. N is the number of oocytes included in each average. Error bars are SEM.J, Effect of amino acid substitutions in the cardiac α1CΔN60 subunit on activation kinetics. Using the same recordings analyzed in I, we plotted the time to reach the half maximal response (t1/2) for a 500 msec depolarizing pulse to +20 mV as the average ± SEM. Error bars are too small to see at this scale for the wild type and some of the mutants. Small, Smaller peak currents resulting from shorter incubation times of oocytes with wild-type cRNA.