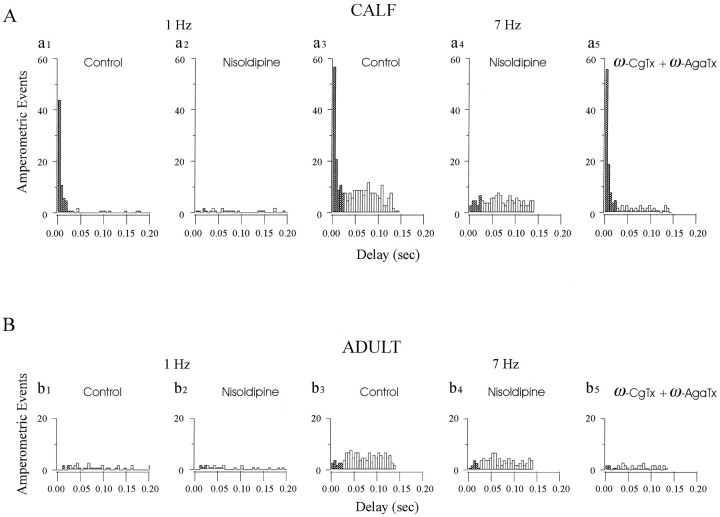
Fig. 5.
Strong stimulus–secretion coupling is attributable to activation of facilitation L-type Ca channels. To determine the proximity of different types of Ca channels to release sites and their contribution to secretion at different frequencies, we analyzed latency histograms from amperometric experiments in the presence of antagonists that selectively block L-type (nisoldipine) or N-type [ω-Conotoxin-GVIA (ω-CgTx)] and P-type [ω-Agatoxin-IVA (ω-AgaTx)] Ca channels. A, Nisoldipine eliminates the strongly coupled peak of secretion at all frequencies in calf AC cells. Latency histograms were derived from the amperometric spikes that resulted from two successive stimulation periods of 1 Hz (a1, a2, 490 APs each) or 7 Hz (a3, a4, 490 APs each) in the absence (Control, a1, a3) or presence of nisoldipine (Nisoldipine, a2, a4). At 7 Hz, nisoldipine suppressed 85% of events with latencies <25 msec and 30% of those with latencies >25 msec after the AP (49% of total events). In parallel experiments (a5, >490 APs), the combination of ω-CgTx and ω-AgaTx had little effect on the short-latency responses (reduced 14% of events <25 msec) but eliminated 77% of events from the weakly coupled plateau (53% of total events). B, Latency histograms derived from adult AC cells using an experimental protocol similar to that shown in A: 1 Hz (b1, b2, 490 APs), 7 Hz (b3, b4, 490 APs) stimulation, each set from the same cell, in the absence (Control, a1 anda3) or presence of nisoldipine (Nisoldipine, b2, b4), or 7 Hz (b5) in the presence of ω-CgTx + ω-AgaTx. At 7 Hz, in comparison with the control, 30 and 69% of total events were suppressed by nisoldipine or the combination of toxins, respectively.