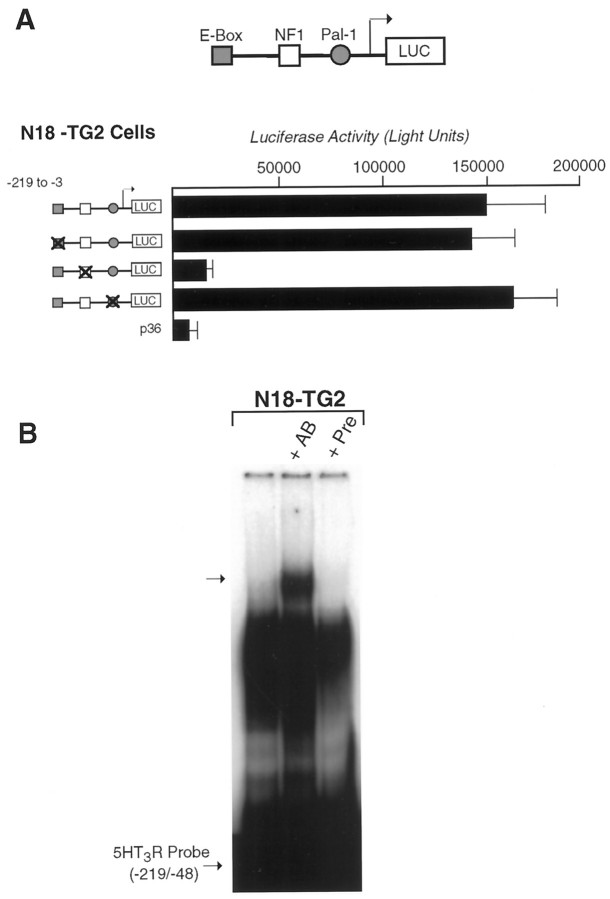
Fig. 5.
Mutation of conserved elements in the5HT3R proximal promoter. A, Individual elements within the 5HT3R proximal promoter were mutated individually and compared with the wild-type promoter in N18-TG2 cell transfection assays. Each mutant promoter constructed is depicted in A, and the exact residues mutated are listed in Table 1. In all cases, either the palindromic nature of the site or the core residues proposed to mediate DNA binding was altered. Loss of the NF1 site results in a 10-fold loss of reporter activity of the 5HT3R proximal promoter and is equivalent to the lowered activity observed with the minimal 5HT3R (−135/−3) reporter construct (Fig. 3A). CMV-βGAL plasmid was cotransfected to control for transfection efficiencies, and the luciferase activity was corrected to reflect these values. B, Super-shift analysis of gel mobility shift assays was performed by preincubating the rabbit anti-chicken NFI antiserum (a gift from Dr U. Kruse, The Scripps Research Institute, La Jolla, CA) or preimmune serum with N18-TG2 nuclear extract before binding to the radiolabeled5HT3R proximal promoter probe. Super-shifted DNA–protein complexes are observed after preincubation with antibody (+AB) but are not present without or with preimmune serum (+Pre) in NG18-TG2 extract.