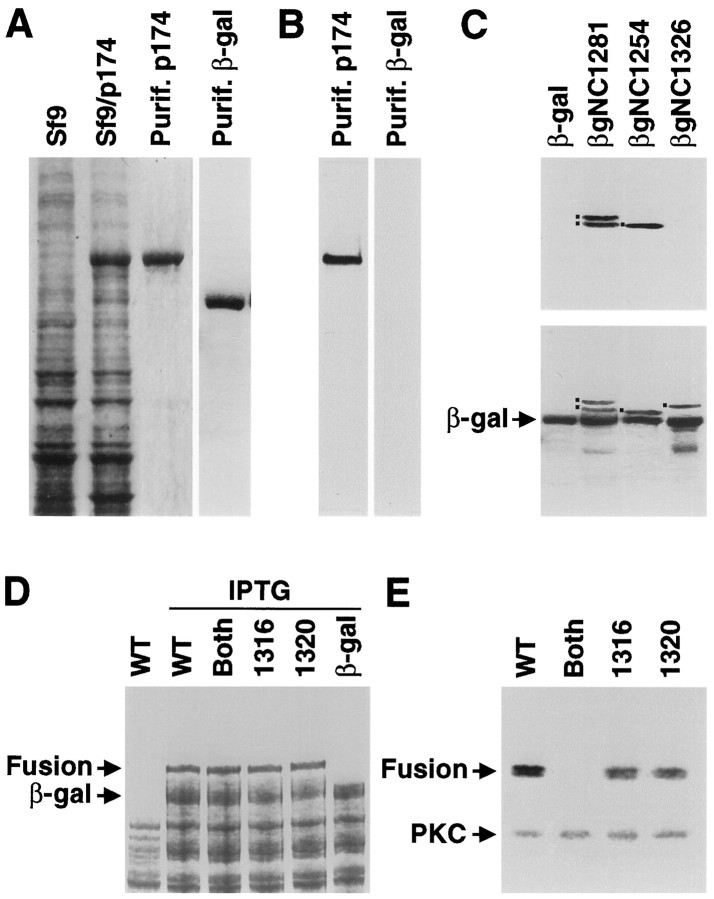
Fig. 3.
p174 is phosphorylated by PKC on serine residues 1316 and 1320. A, p174 expressed in Sf9 cells. Total protein extracts from untransfected Sf9 cells (Sf9), total protein extracts from Sf9 cells transfected with p174 baculovirus-transfected Sf9 cells (Sf9/p174), and p174 purified from Sf9 cells (Purif. p174) were fractionated by SDS-PAGE, and protein was detected by staining with Coomassie blue. In addition, β-galactosidase was expressed inE. coli and purified using p-aminophenyl β-d-thiogalactopyranoside (APTG) beads (Purif. β-gal). B, The purified p174 protein, shown in A, in vitrophosphorylated with PKC and detected by autoradiography. The β-galactosidase, shown in A, did not serve as anin vitro substrate for PKC. C, In vitro phosphorylation of β-galactosidase fusion proteins (see Fig. 2). The fusion proteins (indicated by the dots to the left of the bands) were expressed in BL21 bacteria and labeled with [γ-32P]ATP, and PKC and the fusion proteins were purified with APTG beads.Top, An SDS-PAGE gel stained with Coomassie blue.Bottom, The corresponding autoradiograph. Thelower band in the βgNC1281 lane is a degradation product. D, Expression of derivatives of the βgNC1281 fusion protein in BL21 bacteria. The Coomassie-stained gel shows total bacterial extracts from cells containing unmodified βgNC1281 (WT) minus and plus isopropyl thiogalactoside (IPTG) induction and the total proteins from cells expressing the βgNC1281 derivatives with alanine substitutions at the following sites: both 1316 and 1320 (Both), 1316, and 1320. Total proteins from cells expressing β-galactosidase (β-gal) after IPTG induction are also shown. The fusion protein (Fusion) andβ-gal are indicated. E, PKC phosphorylation assay of βgNC1281 and indicated mutant derivatives. Total bacterial extracts were fractionated by SDS-PAGE, and the phosphoproteins were detected by autoradiography. The fusion proteins (Fusion) and PKC are indicated. The fusion proteins were detected as doublets because of protein degradation that occurred during the course of the phosphorylation assay.