Fig. 4.
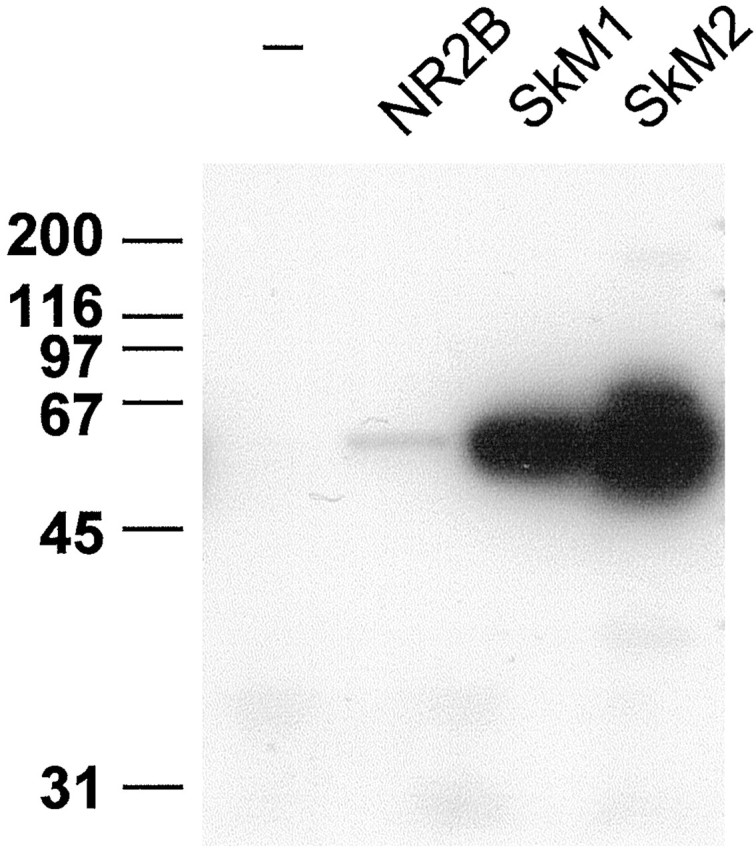
The C termini of muscle NaChs are sufficient for interaction with syntrophins. Approximately equal amounts of biotinylated peptides corresponding to the C-terminal 10 amino acids of the adult skeletal (SkM1) and cardiac (SkM2) muscle NaChs and the NMDA receptor 2B subunit (NR2B) were coupled to streptavidin–agarose beads. Detergent-solubilized heart membranes were incubated with peptide-conjugated beads or with beads alone (−). Bound proteins were immunoblotted for syntrophin. Both the SkM1 and SkM2 peptides bound substantial amounts of syntrophin, whereas NR2B bound much less.
