Fig. 3.
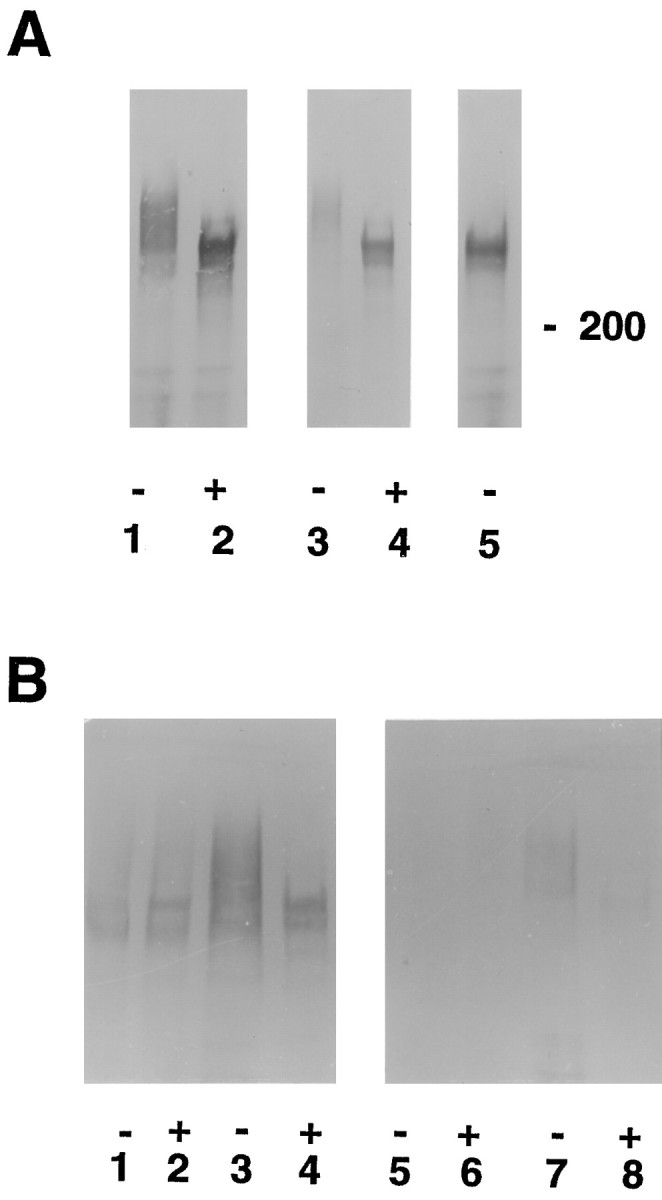
Cat-315 recognizes a CSPG of the same apparent molecular weight in vivo and in vitro, which is primarily produced by neurons. A, Cat-315 immunoprecipitates of conditioned medium from mixed cultures (lanes 1, 2) and cortical homogenates from postnatal day 1 rats (lanes 3,4) incubated in the presence (+) (lanes 2, 4) or absence (-) (lanes 1, 3, 5) of an enzyme that removes chondroitin sulfate glycosaminoglycans were Western blotted and probed with Cat-315. Removal of chondroitin sulfate glycosaminoglycans produces an increase in the mobility of the antigen recognized by Cat-315 (lanes 2, 4), indicating that Cat-315 recognizes a CSPG. The CSPG detected by Cat-315 fromin vivo and in vitro preparations has the same apparent molecular weight both before and after the removal of chondroitin sulfate, suggesting that Cat-315 recognizes the same CSPG in both preparations. For cortical homogenates, each lane was loaded with 25 μg of protein. The deglycosylating enzyme did not produce a shift in mobility of neurofilament (data not shown), which lacks chondroitin sulfate chains, indicating that the increase in mobility on Western blots of the Cat-315 antigen is specifically caused by the removal of glycosaminoglycans and not by proteolysis. Lane 5, To test the efficacy of β-xyloside in prohibiting the addition of glycosaminoglycans, we maintained E16 plus 3 CD cultures in β-xyloside-containing medium for an additional 10 d. Cat-315 immunoprecipitates from conditioned medium from β-xyloside-treated cultures contain an immunoreactive band that migrates to the same molecular weight as the Cat-315 antigen from which chondroitin sulfates have been enzymatically removed. This shows that β-xyloside effectively inhibited glycosaminoglycan addition. B, Cell homogenates (lanes 1, 2,5, 6) and Cat-315 immunoprecipitates of conditioned medium (lanes 3,4, 7, 8) from mixed (lanes 1–4) and pure astrocyte (lanes 5–8) cultures incubated in the presence (+) (lanes 2, 4, 6, 8) or absence (-) (lanes 1, 3,5, 7) of bovine testicular hyaluronidase (which has chondroitinase activity) were Western blotted and probed with Cat-315. Cat-315 immunoreactivity was detected in both cell homogenates and conditioned medium from mixed cultures. Cell homogenates from pure astrocyte cultures were devoid of Cat-315 immunoreactivity; however, trace amounts of Cat-315 immunoreactivity were detected in conditioned medium from these cultures. For cell culture homogenates, each lane was loaded at 50 μg of protein per lane (compare with cortical homogenates in A loaded at 25 μg per lane).
